2.2: Minerals
- Page ID
- 13463
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)The technical definition of a mineral is elegant: a naturally occurring crystalline solid. That definition calls for a bit of commentary, though. By “crystalline” is meant that the atoms of the mineral are arranged in a regular three-dimensional array, called its crystal structure. The qualification “naturally occurring” excludes the multitude of crystalline solids that can be synthesized in the laboratory but are not found in the natural environment. Also, there are naturally occurring solids, like glass or amber, that do not have a crystal structure and therefore technically are not minerals.
There are thousands of named minerals—but, fortunately for us in this course, only a few are common rock-forming minerals, and even fewer figure prominently in the unconsolidated material that mantles the continents.
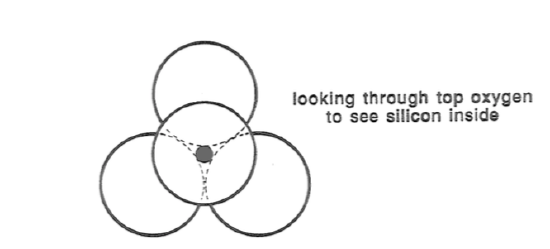
Most of the common minerals are of a class called silicate minerals. Silicate minerals have as their basic building blocks a polyatomic (five-atom) unit, called a silica tetrahedron, that consists of one atom of silicon, relatively small, surrounded by four atoms of oxygen, relatively large, to give the shape of a tetrahedron (Figure 2-3). The five atoms are bonded very strongly together. The reason why silicate minerals are so common is that, in terms of abundances of the chemical elements in the crust, oxygen is the big number one and silicon is second.
The atoms in mineral crystals are held together by strong forces called bonds. An understanding of the nature of bonds necessitates some familiarity with atomic structure. All atoms consist of a positively charged nucleus around which a number of negatively charged electrons are in orbit. The fundamental nature of the electron orbits is complex, but for the purposes of understanding bonds in minerals it’s sufficient to know that electron orbits exist in distinctive groups known as shells.
Chemical elements like helium, neon, or argon whose atoms have just enough electrons to fill all of the shells are chemically almost entirely inert. Elements whose atoms have their outermost electron shell almost filled have a strong tendency to take on one or a few electrons to fill the shell, and in so doing they acquire a negative electric charge. Similarly, elements whose atoms have only one or a few electrons in their outermost shell lose those electrons to revert to a filled-shell configuration, and in so doing they acquire a positive electric charge. Such atoms with electric charges are called ions.
According to what is known in physics as Coulomb’s law, unlike electric charges attract one another and like charges repel one another. Certain combinations of positively charged and negatively charged ions can become packed together in a regular three-dimensional array in such a way that the sum of all the forces, attractive and repulsive, among all of the ions in the structure is attractive, meaning that the structure is a stable one and can exist as a mineral. Almost all minerals are of this nature, and are called ionic crystals; the bonds in such a crystal are called ionic bonds. Ions can also be formed from two or more atoms that share their electrons in such a way that each atoms effectively has a filled-shell configuration. The bonds among such atoms are called covalent bonds. Covalent bonds are much stronger than ionic bonds, and the covalently bonded atoms form a single ion that is available for ionic bonding with other ions.
It turns out that in terms of electric charge the silica tetrahedron has a net negative charge. The silica tetrahedra are bonded to various positively charged atoms, mainly of the elements aluminum, iron, magnesium, calcium, potassium, and sodium. These ionic bonds are weaker than the strong covalent bonds within the silica tetrahedra. When a mineral is broken, it breaks along these ionic bonds rather than through the bonds within the silica tetrahedra.
There is an important further complication to silicate minerals, which makes them highly diverse (and almost unique in the universe!): individual tetrahedra can be joined together by sharing one or more (two, three, or even all four) of their oxygens. The phenomenon is called polymerization. By polymerization, zillions of tetrahedra can be joined into chains or sheets that stretch all the way through a macroscopic mineral grain. In some minerals, all of the oxygens of the tetrahedra are shared, resulting in a complex three-dimensional network of shared tetrahedra.
There are other important minerals besides silicate minerals. Oxide minerals are perhaps the most important kind, in the context of this course. Oxide minerals are those in which negatively charged oxygen atoms are bonded with various positively charged ions. The most important oxides are iron oxides and aluminum oxides. These oxides of iron and aluminum are generally unspectacular in their appearance, but they are voluminous and important in Earth-surface environments. Carbonate minerals are also important near-surface non-silicate minerals. In carbonates, a negatively charged carbonate ion, consisting of one carbon atom covalently bonded with three oxygen atoms, is ionically bonded with calcium, magnesium, and/or iron ions; calcite and dolomite are common carbonate minerals.
Conclusion
To conclude this section, I’ll list just the very most important kinds of minerals in the Earth’s upper crust. It’s important to know something about these minerals, because the mineralogy of solid Earth materials is of great importance in determining how those Earth materials are affected by their physical and chemical environment.
- ferromagnesian silicates (“femags”): This collection of minerals (olivines, pyroxenes, amphiboles, biotite, and certain metamorphic minerals) contain iron and/or magnesium, in various proportions, as their main positive ions. They tend to be dark in color. They are abundant in igneous and metamorphic rocks—but not in sedimentary rocks, because they tend to weather rapidly.
- micas: Micas consist of sheets of polymerized silica tetrahedrons, each with three of their four oxygens shared, with various positive ions between the sheets. The main micas are muscovite (a potassium mica), which is common in igneous, metamorphic, and sedimentary rocks, and biotite (a ferromagnesian mineral, with iron and magnesium rather than potassium). Biotite is common in both metamorphic and igneous rocks, but it weathers rapidly, so it’s uncommon in sediments and sedimentary rocks.
- feldspars: Feldspars are the most abundant kind of mineral in the Earth’s crust. They are network silicates with either potassium (these are called potassium feldspar, or just Kspar for short) or varying proportions of sodium and calcium (these are called plagioclase) in their structure. Feldspars are the major minerals of igneous rocks and are common in many metamorphic rocks. Potassium feldspar is common in sediments and sedimentary rocks, but plagioclase is not, because it weathers readily.
- quartz: Quartz, with the simple formula SiO2, is a network silicate mineral with a three-dimensional network of silica tetrahedra, with all of the oxygens shared with adjacent tetrahedra. It is common but not abundant in igneous rocks, is variably abundant in metamorphic rocks, and is very abundant in sediments and sedimentary rocks.
- iron oxides: Iron oxides, of which there are several forms, are common only in sediments and sedimentary rocks. In soils, there are several poorly crystallized hydrous iron oxide minerals.
- aluminum oxides: In poorly crystallized form, these are characteristic of soils formed by deep weathering of aluminum-bearing rocks under intense weathering conditions.
- carbonates: The calcium carbonate minerals calcite and aragonite, along with the calcium–magnesium mineral dolomite, are the main minerals in chemically precipitated carbonate sedimentary rocks, and they are common as well in sediments in the warm and shallow oceans.
- clay minerals: Several minerals with sheet-silicate structures are common as very fine-grained plate-shaped particles, some even smaller than a micrometer (one-thousandth of a millimeter; too small even to be seen with a powerful optical microscope). Clay minerals are the main constituent of most fine-grained muds.


