8.3: The special properties of water
- Page ID
- 19321
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)A water molecule is composed of one oxygen and two hydrogen atoms that are joined together by polar covalent bonds. Covalent mean that the atoms share electrons, instead of completely giving up electrons to one another. Polar means that the electrons are not shared equally. These polar covalent bonds (Figure 8.2.1), along with the molecular shape, cause the water molecule to have a slightly positive charge on the hydrogen end and a slightly negative charge on the oxygen side. Water’s charges are generated because oxygen is more electronegative than hydrogen, making it more likely that a shared electron would be found near the oxygen nucleus than the hydrogen nucleus, thus generating the partial negative charge near the oxygen. This gives water molecules their properties of attraction.
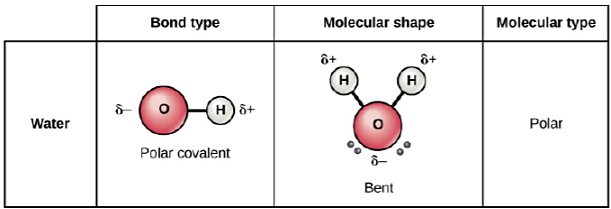.png?revision=1) Figure \(\PageIndex{1}\): Polarity of the water molecule due to the uneven distribution of electrons in its covalent bond. C (From OpenStax Concepts of Biology text)
Figure \(\PageIndex{1}\): Polarity of the water molecule due to the uneven distribution of electrons in its covalent bond. C (From OpenStax Concepts of Biology text)
Hydrogen Bonds
Due to water’s polarity, each water molecule attracts other water molecules as oppositely charged ends of the molecules attract each other. When this happens, a weak interaction occurs between the positive hydrogen end from one molecule and the negative oxygen end of another molecule. This interaction is called a hydrogen bond. This hydrogen bonding contributes to the following water’s unique properties.
1. Water is the universal solvent
2. Exists in nature as a solid, liquid, and gas
3. The density of ice is less than liquid water
4. Water has a high surface tension
5. Water has a high heat capacity
6. Water exists as a liquid at room temperature It is important to note here that even we are only focusing on water in this text book, hydrogen bonding also occurs in other substances that have polar molecules.
Physical State of Water on Earth
Water on Earth can naturally exist as either solid, liquid or gas depending on the prevailing temperature and pressure conditions. The formation of hydrogen bonds (described above) is an important quality of liquid water that is crucial to life as we know it on Earth. As water molecules make hydrogen bonds with each other, liquid water takes on some unique physical and chemical characteristics when compared to other liquids. In liquid water, hydrogen bonds are constantly being formed and broken as the water molecules slide past each other. The energy of the moving water molecules (kinetic energy) is responsible for breaking the bonds. When heat is added to water (increasing temperature), the kinetic energy of the molecules goes up and more bonds are broken. As more heat is added to boiling water, the higher kinetic energy of the water molecules causes the hydrogen bonds to break completely and allow them to escape into the air as water vapor. On the other hand, when the temperature of water is reduced and water freezes, the water molecules form a crystalline structure maintained by hydrogen bonding (since there isn’t enough energy to break the hydrogen bonds). The crystalline structure, ice, has a more open structure than the liquid form of water. The open structure of ice (Figure \(\PageIndex{2}\)) makes ice less dense than liquid water, a phenomenon not seen in the solidification of other liquids.
The lower density of ice, illustrated in Figure \(\PageIndex{2}\), causes it to float at the surface of liquid water, such as an iceberg in the ocean or ice cubes in a glass of ice water. In lakes and ponds, ice will form on the surface of water creating an insulating barrier that protects animals and plant life that live in the water from freezing. Without this layer of insulating ice, plants and animals living in the water would freeze in the solid block of ice and not survive. The ice crystals that form upon freezing would rupture the delicate membranes essential for the function of living cells, irreversibly damaging them.
.png?revision=1) Figure \(\PageIndex{2}\): Hydrogen bonding makes ice less dense than liquid water. The lattice structure water is more condensed (left structure) than that of ice (right structure). The lattice structure of ice makes it less dense than freely flowing molecules of liquid water, enabling ice to float on liquid water. ( Image credit: Lynn Yarris, http://www2.lbl.gov/Science-Articles...ter-solid.html)
Figure \(\PageIndex{2}\): Hydrogen bonding makes ice less dense than liquid water. The lattice structure water is more condensed (left structure) than that of ice (right structure). The lattice structure of ice makes it less dense than freely flowing molecules of liquid water, enabling ice to float on liquid water. ( Image credit: Lynn Yarris, http://www2.lbl.gov/Science-Articles...ter-solid.html)
High Heat Capacity
Water has the highest specific heat capacity of any liquid. Water’s high heat capacity is a property caused by hydrogen bonding among the water molecules. Specific heat is defined as the amount of heat one gram of a substance must absorb or lose to change its temperature by one degree Celsius. For water, this amount is one calorie. It takes water a long time to heat up and a long time to cool down. In fact, the specific heat capacity of water is about five times more than that of sand. This explains why land cools faster than the sea. Due to its high heat capacity, warm-blooded animals use water to disperse heat more evenly and maintain temperature in their bodies: it acts in a similar manner to a car’s cooling system, transporting heat from warm places to cool places, causing the body to maintain a more even temperature.
Heat of Vaporization
Water also has a high heat of vaporization, the amount of energy required to change one gram of a liquid substance to a gas. A considerable amount of heat energy (586 calories) is required to accomplish this change in water. This process occurs on the surface of water. As liquid water heats up, hydrogen bonding makes it difficult to separate the liquid water molecules from each other, which is required for it to enter the gas phase (steam). Thus, water acts as a heat sink and requires much more heat to boil than liquids such as ethanol, whose hydrogen bonds are weaker. Eventually, as water reaches its boiling point of 100° Celsius (212° Fahrenheit), the heat can break the hydrogen bonds between the water molecules, and the kinetic energy between the water molecules allows them to escape from the liquid as a gas. Even when below its boiling point, water’s individual molecules acquire enough energy from other water molecules such that some surface water molecules can escape and vaporize: this process is known as evaporation.
Since hydrogen bonds need to be broken for water to evaporate means that a substantial amount of energy is used in the evaporation process. As the water evaporates, energy is taken up by the process, cooling the environment where the evaporation is taking place. In many living organisms, including in humans, the evaporation of sweat, which is 90 percent water, allows the organism to cool so that homeostasis of body temperature can be maintained.
Water is a Solvent
Since water is a polar molecule with slightly positive and slightly negative charges, ions and polar molecules can readily dissolve in it. Water is, therefore, referred to as a solvent, because it is capable of dissolving more substances (polar substances) than any other liquid. The charges associated with these molecules will form hydrogen bonds with water, surrounding the particle with water molecules. This is very important as it enables water to dissolve various chemicals and distribute them within living organisms, including taking toxic substances out of living things, and in the environment.
Water’s Cohesive and Adhesive Properties
Have you ever filled a glass of water to the very top and then slowly added a few more drops? Before it overflows, the water forms a dome-like shape above the rim of the glass (Figure \(\PageIndex{3}\))
.png?revision=1) Figure \(\PageIndex{3}\): Water in a glass form a dome shape above the glass due to cohesive forces of attraction among water molecules. Photo Credit: Sam Mutiti
Figure \(\PageIndex{3}\): Water in a glass form a dome shape above the glass due to cohesive forces of attraction among water molecules. Photo Credit: Sam Mutiti
This water can stay above the glass because of its cohesive properties. In cohesion, water molecules are attracted to each other (because of hydrogen bonding), keeping the molecules together at the liquid-gas (water-air) interface, although there is no more room in the glass. Cohesion allows for the development of surface tension, the capacity of a substance to resist rupture when placed under tension or stress. This is also why water forms droplets when placed on a dry surface rather than being flattened out by gravity (Figure \(\PageIndex{3}\))
.png?revision=1) Figure \(\PageIndex{4}\): Beading up of water due strong cohesive forces between water molecules (Water USGS, right hand photo credit: J Schmidt; National Park Service).
Figure \(\PageIndex{4}\): Beading up of water due strong cohesive forces between water molecules (Water USGS, right hand photo credit: J Schmidt; National Park Service).
When a steel needle is placed carefully on water it does not sink even though steel is denser (heavier) than the water. Cohesion and surface tension keep the hydrogen bonds of water molecules intact and support the item floating on the top. It is even possible for an insect to “float” on water if it sits gently without breaking the surface tension, as shown in Figure \(\PageIndex{5}\).
.png?revision=1) Figure \(\PageIndex{5}\): The weights of the needle and water strider are pulling the surface downward; at the same time, the surface tension is pulling it up, suspending them on the surface of the water and keeping them from sinking. (Credit: Cory Zanker (left) and Tim Vickers (right)
Figure \(\PageIndex{5}\): The weights of the needle and water strider are pulling the surface downward; at the same time, the surface tension is pulling it up, suspending them on the surface of the water and keeping them from sinking. (Credit: Cory Zanker (left) and Tim Vickers (right)
Another important property of water is adhesion, or the attraction between water molecules and other molecules. This attraction is sometimes stronger than water’s cohesive forces, especially when water is exposed to charged surfaces such as on the inside of thin glass tubes known as capillary tubes. Adhesion is observed when water “climbs” up the tube placed in a glass of water: notice that the water appears to be higher on the sides of the tube than in the middle. This is because the water molecules are attracted to the charged glass walls of the capillary tube more than they are to each other and, therefore, adhere to it. This type of adhesion is called capillary action, and is illustrated in Figure \(\PageIndex{6}\). This process is also involved in the movement of water and nutrients from the soil around the root systems to other parts of plants above the ground.
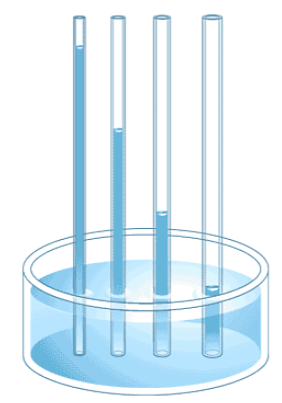.png?revision=1&size=bestfit&width=229&height=327) Figure \(\PageIndex{6}\): Capillary action in a glass tube is caused by the adhesive forces exerted by the internal surface of the glass exceeding the cohesive forces between the water molecules themselves. (Credit: moodle.clsd.k12.pa.us/distric...s/0-13-115540- 7/ch23/ch23_s5_1.html)
Figure \(\PageIndex{6}\): Capillary action in a glass tube is caused by the adhesive forces exerted by the internal surface of the glass exceeding the cohesive forces between the water molecules themselves. (Credit: moodle.clsd.k12.pa.us/distric...s/0-13-115540- 7/ch23/ch23_s5_1.html)

