2.1: Biogeochemical Cycles
- Page ID
- 11744
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)One way to gain a broader understanding of sustainable systems is to look for examples in nature. Ecosystems provide just such a model because they have persisted for very long periods of time. When we think of ecosystems, we tend to conjure images of animals in some kind of natural environment, such as a forest or savannah. Indeed, the study of ecosystems entails an effort to understand the way all of the organisms inhabiting a given area interact with one another.
However, there are two underlying concepts that are present in all ecosystems. First, they are all systems that cycle matter. Second, energy flows through them all. You might even say that the idea of an ecosystem that we see at face value, with its predators attacking prey, animals grazing on grass and so forth, is really just a complex mechanism for cycling matter and facilitating the flow of energy.
There are important principles that apply to these concepts: the Law of Conservation of Matter and the two Laws of Thermodynamics. In this section, we will cover matter and energy in detail. These ideas will provide a foundation for understanding many environmental issues as well as the elusive concept of sustainability.
Cycling of Matter
Energy flows directionally through ecosystems, entering as sunlight (or inorganic molecules for chemoautotrophs) and leaving as heat during the transfers between trophic levels (covered in the Ecology Section). Rather than flowing through an ecosystem, the matter that makes up living organisms is conserved and recycled. This is the essence of the Law of Conservation of Matter. The six most common elements associated with organic molecules—carbon, nitrogen, hydrogen, oxygen, phosphorus, and sulfur—take a variety of chemical forms and may exist for long periods in the atmosphere, on land, in water, or beneath Earth’s surface. Geologic processes, such as weathering, erosion, water drainage, and the subduction of the continental plates, all play a role in the cycling of elements on Earth. Because geology and chemistry have major roles in the study of this process, the recycling of inorganic matter between living organisms and their nonliving environment is called a biogeochemical cycle.
Water, which contains hydrogen and oxygen, is essential to all living processes. The hydrosphere is the area of Earth where water movement and storage occurs: as liquid water on the surface (rivers, lakes, oceans) and beneath the surface (groundwater) or ice, (polar ice caps and glaciers), and as water vapor in the atmosphere. Carbon is found in all organic macromolecules and is an important constituent of fossil fuels. Nitrogen is a major component of our nucleic acids and proteins and is critical to human agriculture. Phosphorus, a major component of nucleic acids, is one of the main ingredients (along with nitrogen) in artificial fertilizers used in agriculture, which has environmental impacts on our surface water. Sulfur, critical to the three-dimensional folding of proteins (as in disulfide binding), is released into the atmosphere by the burning of fossil fuels.
The cycling of these elements is interconnected. For example, the movement of water is critical for the leaching of nitrogen and phosphate into rivers, lakes, and oceans. The ocean is also a major reservoir for carbon. Thus, mineral nutrients are cycled, either rapidly or slowly, through the entire biosphere between the biotic and abiotic world and from one living organism to another.
The Water Cycle
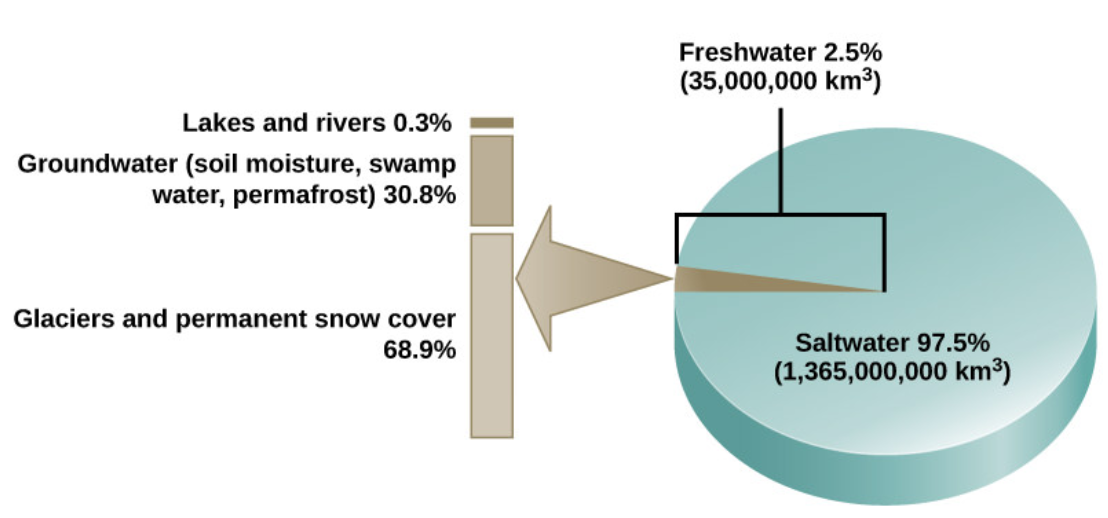
Water is essential for all living processes. The human body is more than one-half water and human cells are more than 70 percent water. Thus, most land animals need a supply of fresh water to survive. Of the stores of water on Earth, 97.5 percent is salt water (Figure \(\PageIndex{1}\)). Of the remaining water, 99 percent is locked as underground water or ice. Thus, less than one percent of fresh water is present in lakes and rivers. Many living things are dependent on this small amount of surface fresh water supply, a lack of which can have important effects on ecosystem dynamics. Humans, of course, have developed technologies to increase water availability, such as digging wells to harvest groundwater, storing rainwater, and using desalination to obtain drinkable water from the ocean. Although this pursuit of drinkable water has been ongoing throughout human history, the supply of fresh water continues to be a major issue in modern times.
The various processes that occur during the cycling of water are illustrated in Figure below. The processes include the following:
- evaporation and sublimation
- condensation and precipitation
- subsurface water flow
- surface runoff and snowmelt
- streamflow
The water cycle is driven by the Sun’s energy as it warms the oceans and other surface waters. This leads to evaporation (water to water vapor) of liquid surface water and sublimation (ice to water vapor) of frozen water, thus moving large amounts of water into the atmosphere as water vapor. Over time, this water vapor condenses into clouds as liquid or frozen droplets and eventually leads to precipitation (rain or snow), which returns water to Earth’s surface. Rain reaching Earth’s surface may evaporate again, flow over the surface (runoff), or percolate into the ground (infiltration). Most easily observed is surface runoff: the flow of fresh water either from rain or melting ice. Runoff can make its way through streams and lakes to the oceans or flow directly to the oceans themselves.
In most natural terrestrial environments rain encounters vegetation before it reaches the soil surface. A significant percentage of water evaporates immediately from the surfaces of plants. What is left reaches the soil and begins to move down. Surface runoff will occur only if the soil becomes saturated with water in a heavy rainfall. Most water in the soil will be taken up by plant roots. The plant will use some of this water for its own metabolism, and some of that will find its way into animals that eat the plants, but much of it will be lost back to the atmosphere through a process known as evapotranspiration. Water enters the vascular system of the plant through the roots and evaporates, or transpires, through the stomata (small openings for gas exchange) of the leaves. Water in the soil that is not taken up by a plant and that does not evaporate is able to percolate into the subsoil and bedrock. Here it forms groundwater.
Groundwater is a significant reservoir of fresh water. It exists in the pores between particles in sand and gravel, or in the fissures in rocks. Shallow groundwater flows slowly through these pores and fissures and eventually finds its way to a stream or lake where it becomes a part of the surface water again. Streams do not flow because they are replenished from rainwater directly; they flow because there is a constant inflow from groundwater below. Some groundwater is found very deep in the bedrock and can persist there for millennia. Most groundwater reservoirs, or aquifers, are the source of drinking or irrigation water drawn up through wells. In many cases these aquifers are being depleted faster than they are being replenished by water percolating down from above.
Rain and surface runoff are major ways in which minerals, including carbon, nitrogen, phosphorus, and sulfur, are cycled from land to water. The environmental effects of runoff will be discussed later as these cycles are described.
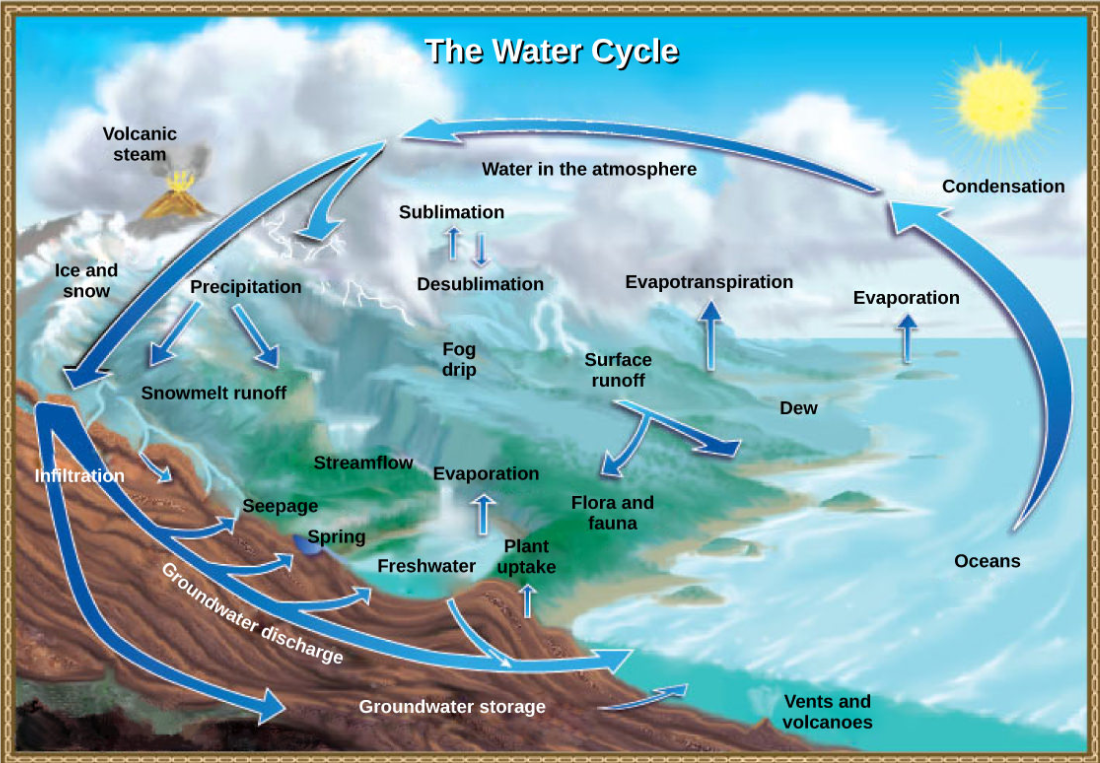
Figure \(\PageIndex{2}\): Water from the land and oceans enters the atmosphere by evaporation or sublimation, where it condenses into clouds and falls as rain or snow. Precipitated water may enter freshwater bodies or infiltrate the soil. The cycle is complete when surface or groundwater reenters the ocean. (credit: modification of work by John M. Evans and Howard Perlman, USGS)
The Carbon Cycle
Carbon is the fourth most abundant element in living organisms. Carbon is present in all organic molecules, and its role in the structure of macromolecules is of primary importance to living organisms. Carbon compounds contain energy, and many of these compounds from plants and algae have remained stored as fossilized carbon, which humans use as fuel. Since the 1800s, the use of fossil fuels has accelerated. As global demand for Earth’s limited fossil fuel supplies has risen since the beginning of the Industrial Revolution, the amount of carbon dioxide in our atmosphere has increased as the fuels are burned. This increase in carbon dioxide has been associated with climate change and is a major environmental concern worldwide.
The carbon cycle is most easily studied as two interconnected subcycles: one dealing with rapid carbon exchange among living organisms and the other dealing with the long-term cycling of carbon through geologic processes. The entire carbon cycle is shown in Figure below.
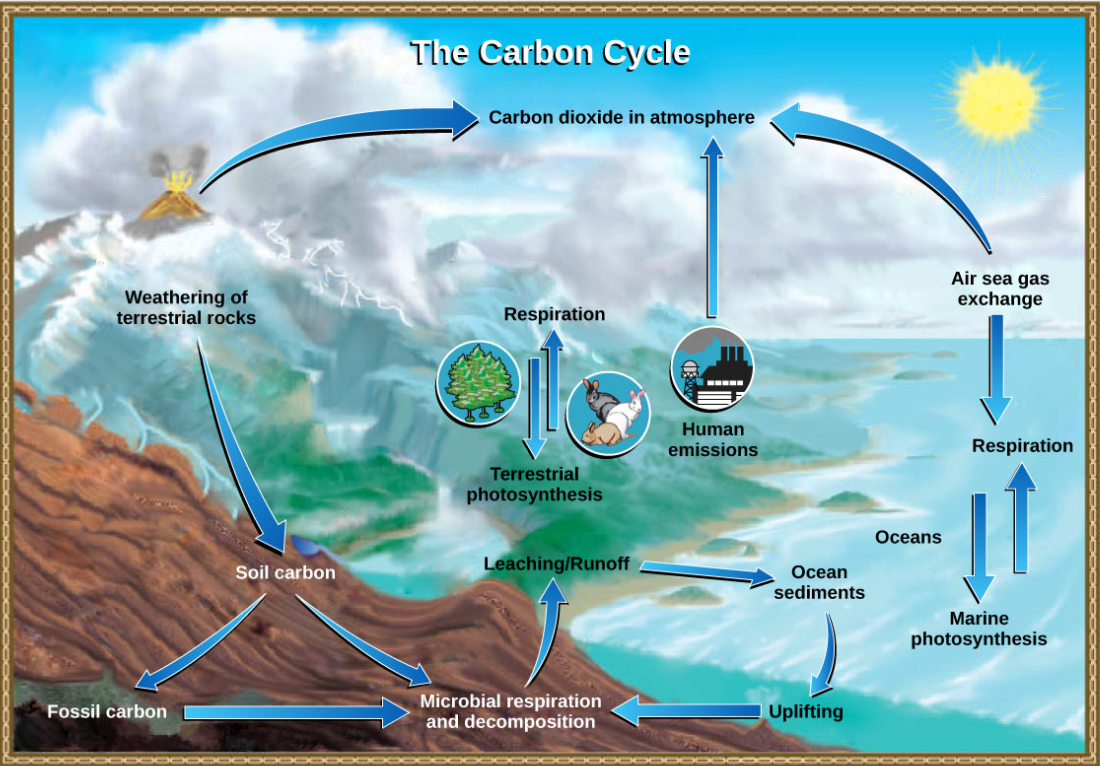
Figure \(\PageIndex{3}\): Carbon dioxide gas exists in the atmosphere and is dissolved in water. Photosynthesis converts carbon dioxide gas to organic carbon, and respiration cycles the organic carbon back into carbon dioxide gas. Long-term storage of organic carbon occurs when matter from living organisms is buried deep underground and becomes fossilized. Volcanic activity and, more recently, human emissions bring this stored carbon back into the carbon cycle. (credit: modification of work by John M. Evans and Howard Perlman, USGS)
The Biological Carbon Cycle
Living organisms are connected in many ways, even between ecosystems. A good example of this connection is the exchange of carbon between heterotrophs (those that must consume food) and autotrophs (those that photosynthesize) within and between ecosystems by way of atmospheric carbon dioxide. Carbon dioxide is the basic building block that autotrophs use to build multi-carbon, high-energy compounds, such as glucose. The energy harnessed from the Sun is used by these organisms to form the bonds that link carbon atoms together. These chemical bonds store this energy for later use in the process of respiration. Most terrestrial autotrophs obtain their carbon dioxide directly from the atmosphere, while marine autotrophs acquire it in the dissolved form (carbonic acid, \(\ce{HCO3^-}\)). However the carbon dioxide is acquired, a byproduct of fixing carbon in organic compounds is oxygen. Photosynthetic organisms are responsible for maintaining approximately 21 percent of the oxygen content of the atmosphere that we observe today.
The partners in biological carbon exchange are the heterotrophs (especially the primary consumers, largely herbivores). Heterotrophs acquire the high-energy carbon compounds from the autotrophs by consuming them and breaking them down by respiration to obtain cellular energy. Thus, there is a constant exchange of oxygen and carbon dioxide between the autotrophs (which need the carbon) and the heterotrophs (which need the oxygen). Autotrophs also respire and consume the organic molecules they form: using oxygen and releasing carbon dioxide. They release more oxygen gas as a waste product of photosynthesis than they use for their own respiration; therefore, there is excess available for the respiration of other aerobic organisms. Gas exchange through the atmosphere and water is one way that the carbon cycle connects all living organisms on Earth.
The Biogeochemical Carbon Cycle
The movement of carbon through land, water, and air is complex, and, in many cases, it occurs much more slowly geologically than the movement between living organisms. Carbon is stored for long periods in what are known as carbon reservoirs (or carbon sinks), which include the atmosphere, bodies of liquid water (mostly oceans), ocean sediment, soil, rocks (including fossil fuels), and Earth’s interior.
As stated, the atmosphere is a major reservoir of carbon in the form of carbon dioxide that is essential to the process of photosynthesis. The level of carbon dioxide in the atmosphere is greatly influenced by the reservoir of carbon in the oceans. The exchange of carbon between the atmosphere and water reservoirs influences how much carbon is found in each, and each one affects the other reciprocally. Carbon dioxide (\(\ce{CO2}\)) from the atmosphere dissolves in water and, unlike oxygen and nitrogen gas, reacts with water molecules to form ionic compounds. Some of these ions combine with calcium ions in the seawater to form calcium carbonate (\(\ce{CaCO3}\)), a major component of the shells of marine organisms. These organisms eventually form sediments on the ocean floor. Over geologic time, the calcium carbonate forms limestone, which comprises the largest carbon reservoir on Earth. Increases in atmospheric carbon dioxide also increase the amount absorbed by oceans leading to ocean acidification.
On land, carbon is stored in soil as organic carbon as a result of the decomposition of living organisms or from weathering of terrestrial rock and minerals. Deeper under the ground, at land and at sea, are fossil fuels, the anaerobically decomposed remains of plants that take millions of years to form. Fossil fuels are considered a non-renewable resource because their use far exceeds their rate of formation. A non-renewable resource is either regenerated very slowly or not at all. Another way for carbon to enter the atmosphere is from land (including land beneath the surface of the ocean) by the eruption of volcanoes and other geothermal systems. Carbon sediments from the ocean floor are taken deep within Earth by the process of subduction: the movement of one tectonic plate beneath another. Carbon is released as carbon dioxide when a volcano erupts or from volcanic hydrothermal vents.
Carbon dioxide is also added to the atmosphere by the animal husbandry practices of humans. The large number of land animals raised to feed Earth’s growing human population results in increased carbon-dioxide levels in the atmosphere caused by their respiration. This is another example of how human activity indirectly affects biogeochemical cycles in a significant way. Although much of the debate about the future effects of increasing atmospheric carbon on climate change focuses on fossils fuels, scientists take natural processes, such as volcanoes, plant growth, soil carbon levels, and respiration, into account as they model and predict the future impact of this increase.
The Nitrogen Cycle
Getting nitrogen into living organisms is difficult. Plants and phytoplankton are not equipped to incorporate nitrogen from the atmosphere (where it exists as tightly bonded, triple covalent \(\ce{N2}\)) even though this molecule comprises approximately 78 percent of the atmosphere. Nitrogen enters the living world through free-living and symbiotic bacteria, which incorporate nitrogen into their macromolecules through specialized biochemical pathways leading to nitrogen fixation. Cyanobacteria live in most aquatic ecosystems where sunlight is present; they play a key role in nitrogen fixation. Cyanobacteria are able to “fix” nitrogen (from nitrogen gas) into ammonia (\(\ce{NH3}\)) that can be incorporated into the macromolecules of the organism. Rhizobium bacteria also fix nitrogen and live symbiotically in the root nodules of legumes (such as peas, beans, and peanuts) and provide them with the organic nitrogen they need. Free-living bacteria, such as Azotobacter, are also able to fix nitrogen.
Organic nitrogen is especially important to the study of ecosystem dynamics since many ecosystem processes, such as primary production and decomposition, are limited by the available supply of nitrogen. As shown in Figure below, the nitrogen that enters living systems by nitrogen fixation is eventually converted from organic nitrogen back into nitrogen gas by bacteria. This process occurs in three steps in terrestrial systems: ammonification, nitrification, and denitrification. First, the ammonification process converts nitrogenous waste from living animals or from the remains of dead animals into ammonium (\(\ce{NH4^+}\)) by certain bacteria and fungi. Second, this ammonium is then converted to nitrites (\(\ce{NO2^-}\)) by nitrifying bacteria, such as Nitrosomonas, through nitrification. Subsequently, nitrites are converted to nitrates (\(\ce{NO3^-}\)) by similar organisms. Lastly, the process of denitrification occurs, whereby bacteria, such as Pseudomonas and Clostridium, convert the nitrates into nitrogen gas, thus allowing it to re-enter the atmosphere.
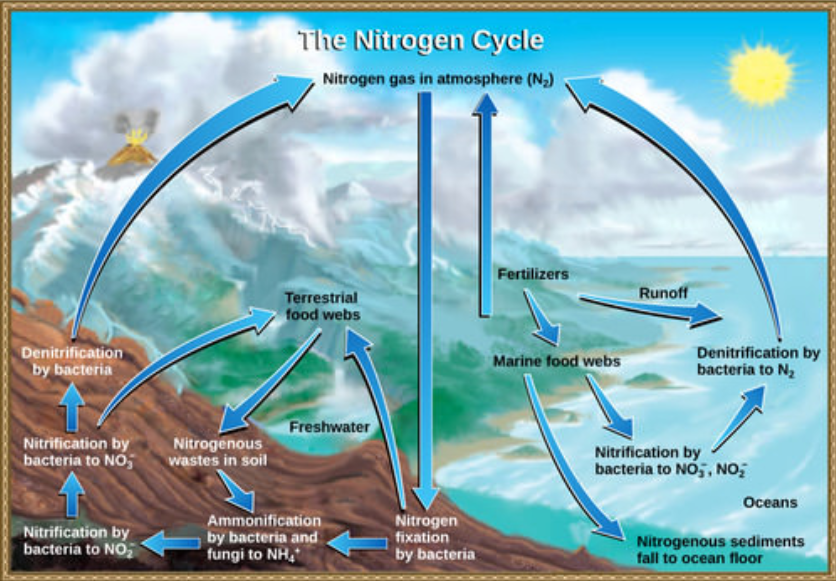
Figure \(\PageIndex{4}\)Nitrogen enters the living world from the atmosphere through nitrogen-fixing bacteria. This nitrogen and nitrogenous waste from animals is then processed back into gaseous nitrogen by soil bacteria, which also supply terrestrial food webs with the organic nitrogen they need. (credit: modification of work by John M. Evans and Howard Perlman, USGS
Human activity can release nitrogen into the environment by two primary means: the combustion of fossil fuels, which releases different nitrogen oxides, and by the use of artificial fertilizers (which contain nitrogen and phosphorus compounds) in agriculture, which are then washed into lakes, streams, and rivers by surface runoff. Atmospheric nitrogen (other than \(\ce{N2}\)) is associated with several effects on Earth’s ecosystems including the production of acid rain (as nitric acid, \(\ce{HNO3}\)) and greenhouse gas effects (as nitrous oxide, \(\ce{N2O}\)), which drive climate change. A major effect from fertilizer runoff is saltwater and freshwater eutrophication, a process whereby nutrient runoff causes the overgrowth of algae, the depletion of oxygen and death of aquatic fauna.
A similar process occurs in the marine nitrogen cycle, where the ammonification, nitrification, and denitrification processes are performed by marine bacteria and archaea. Some of this nitrogen falls to the ocean floor as sediment, which can then be moved to land in geologic time by uplift of Earth’s surface, and thereby incorporated into terrestrial rock. Although the movement of nitrogen from rock directly into living systems has been traditionally seen as insignificant compared with nitrogen fixed from the atmosphere, a recent study showed that this process may indeed be significant and should be included in any study of the global nitrogen cycle.
The Phosphorus Cycle
Phosphorus is an essential nutrient for living processes; it is a major component of nucleic acids and phospholipids, and, as calcium phosphate, makes up the supportive components of our bones. Phosphorus is often the limiting nutrient (necessary for growth) in aquatic, particularly freshwater, ecosystems.
Phosphorus occurs in nature as the phosphate ion (\(\ce{PO4^3-}\)). In addition to phosphate runoff as a result of human activity, natural surface runoff occurs when it is leached from phosphate-containing rock by weathering, thus sending phosphates into rivers, lakes, and the ocean. This rock has its origins in the ocean. Phosphate-containing ocean sediments form primarily from the bodies of ocean organisms and from their excretions. However, volcanic ash, aerosols, and mineral dust may also be significant phosphate sources. This sediment then is moved to land over geologic time by the uplifting of Earth’s surface. (Figure below)
Phosphorus is also reciprocally exchanged between phosphate dissolved in the ocean and marine organisms. The movement of phosphate from the ocean to the land and through the soil is extremely slow, with the average phosphate ion having an oceanic residence time between 20,000 and 100,000 years.
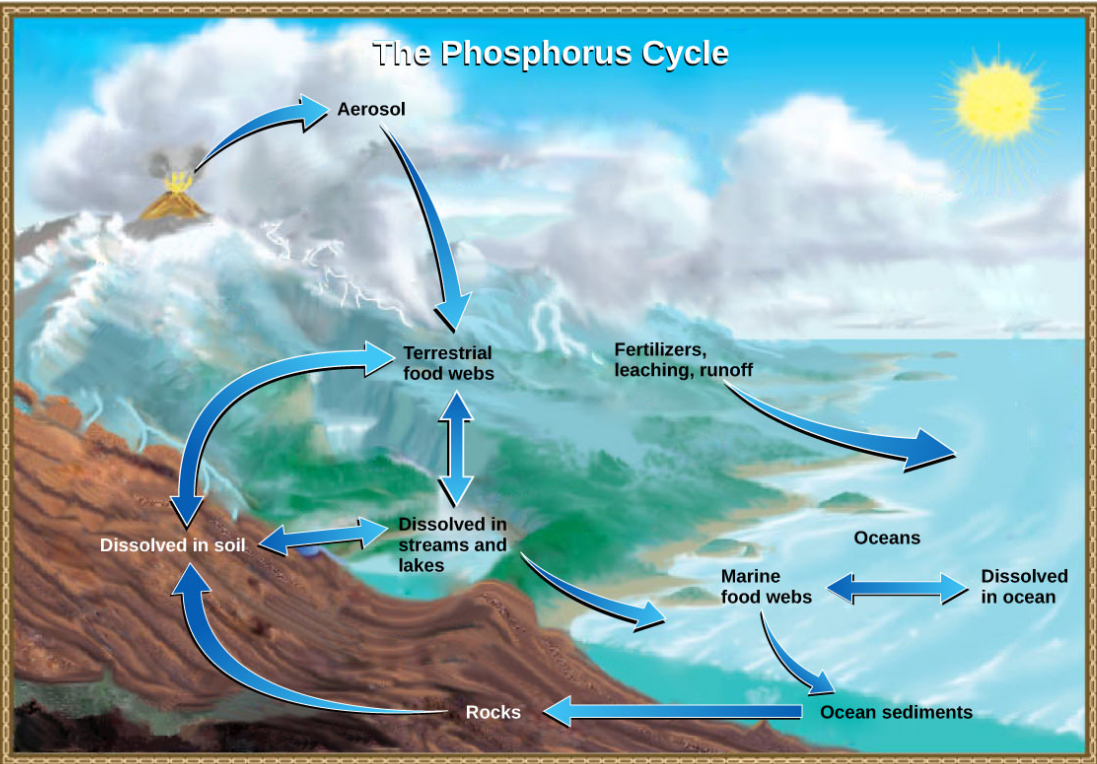
Figure \(\PageIndex{5}\): In nature, phosphorus exists as the phosphate ion (\(\ce{PO4^3-}\)). Weathering of rocks and volcanic activity releases phosphate into the soil, water, and air, where it becomes available to terrestrial food webs. Phosphate enters the oceans in surface runoff, groundwater flow, and river flow. Phosphate dissolved in ocean water cycles into marine food webs. Some phosphate from the marine food webs falls to the ocean floor, where it forms sediment. (credit: modification of work by John M. Evans and Howard Perlman, USGS)
Excess phosphorus and nitrogen that enter these ecosystems from fertilizer runoff and from sewage cause excessive growth of algae. The subsequent death and decay of these organisms depletes dissolved oxygen, which leads to the death of aquatic organisms, such as shellfish and fish. This process is responsible for dead zones in lakes and at the mouths of many major rivers and for massive fish kills, which often occur during the summer months (see Figure below).
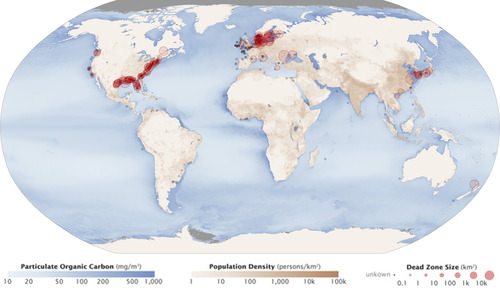
A dead zone is an area in lakes and oceans near the mouths of rivers where large areas are periodically depleted of their normal flora and fauna; these zones are caused by eutrophication coupled with other factors including oil spills, dumping toxic chemicals, and other human activities. The number of dead zones has increased for several years, and more than 400 of these zones were present as of 2008. One of the worst dead zones is off the coast of the United States in the Gulf of Mexico: fertilizer runoff from the Mississippi River basin created a dead zone of over 8,463 square miles. Phosphate and nitrate runoff from fertilizers also negatively affect several lake and bay ecosystems including the Chesapeake Bay in the eastern United States.
The Sulfur Cycle
Sulfur is an essential element for the macromolecules of living things. As part of the amino acid cysteine, it is involved in the formation of proteins. As shown in Figure below, sulfur cycles between the oceans, land, and atmosphere. Atmospheric sulfur is found in the form of sulfur dioxide (\(\ce{SO2}\)), which enters the atmosphere in three ways: first, from the decomposition of organic molecules; second, from volcanic activity and geothermal vents; and, third, from the burning of fossil fuels by humans.
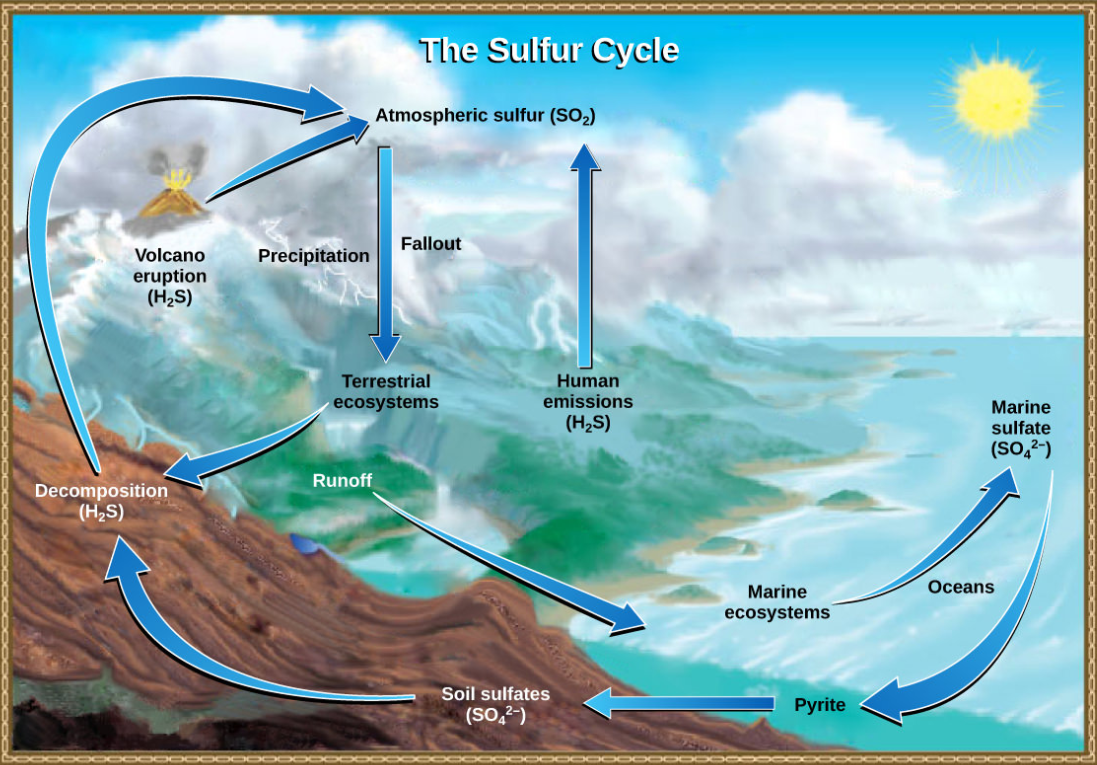
Figure \(\PageIndex{7}\): Sulfur dioxide from the atmosphere becomes available to terrestrial and marine ecosystems when it is dissolved in precipitation as weak sulfuric acid or when it falls directly to Earth as fallout. Weathering of rocks also makes sulfates available to terrestrial ecosystems. Decomposition of living organisms returns sulfates to the ocean, soil, and atmosphere. (credit: modification of work by John M. Evans and Howard Perlman, USGS)
On land, sulfur is deposited in four major ways: precipitation, direct fallout from the atmosphere, rock weathering, and geothermal vents. Atmospheric sulfur is found in the form of sulfur dioxide (\(\ce{SO2}\)), and as rain falls through the atmosphere, sulfur is dissolved in the form of weak sulfuric acid (\(\ce{H2SO4}\)). Sulfur can also fall directly from the atmosphere in a process called fallout. Also, as sulfur-containing rocks weather, sulfur is released into the soil. These rocks originate from ocean sediments that are moved to land by the geologic uplifting of ocean sediments. Terrestrial ecosystems can then make use of these soil sulfates (\(\ce{SO4^2-}\)), which enter the food web by being taken up by plant roots. When these plants decompose and die, sulfur is released back into the atmosphere as hydrogen sulfide (\(\ce{H2S}\)) gas.
Sulfur enters the ocean in runoff from land, from atmospheric fallout, and from underwater geothermal vents. Some ecosystems rely on chemoautotrophs using sulfur as a biological energy source. This sulfur then supports marine ecosystems in the form of sulfates.
Human activities have played a major role in altering the balance of the global sulfur cycle. The burning of large quantities of fossil fuels, especially from coal, releases larger amounts of hydrogen sulfide gas into the atmosphere. As rain falls through this gas, it creates the phenomenon known as acid rain, which damages the natural environment by lowering the pH of lakes, thus killing many of the resident plants and animals. Acid rain is corrosive rain caused by rainwater falling to the ground through sulfur dioxide gas, turning it into weak sulfuric acid, which causes damage to aquatic ecosystems. Acid rain also affects the man-made environment through the chemical degradation of buildings. For example, many marble monuments, such as the Lincoln Memorial in Washington, DC, have suffered significant damage from acid rain over the years. These examples show the wide-ranging effects of human activities on our environment and the challenges that remain for our future.

