15.2: Fossil Fuels
- Page ID
- 32262
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Fossils fuels are extractable sources of stored energy created by ancient ecosystems. The natural resources that typically fall under this category are coal, oil (petroleum), and natural gas. These resources were originally formed via photosynthesis by living organisms such as plants, phytoplankton, algae, and cyanobacteria. This energy is actually fossil solar energy, since the Sun’s ancient energy was converted by ancient organisms into tissues that preserved the chemical energy within the fossil fuel. Of course, as the energy is used, just like respiration from photosynthesis that occurs today, carbon can enter the atmosphere as CO2, causing climate consequences. Fossil fuels account for a large portion of the energy used in the world.
![<a data-cke-saved-href="https://en.Wikipedia.org/wiki/User:Staplegunther" href="https://en.Wikipedia.org/wiki/User:Staplegunther" class="extiw" title="en:User:Staplegunther Staplegunther at the English language Wikipedia [GFDL or CC-BY-SA-3.0], via Wikimedia Commons The power plant has smoke coming from it](https://geo.libretexts.org/@api/deki/files/33337/16.2_Castle_Gate_Power_Plant_Utah_2007-300x188.jpg?revision=1&size=bestfit&width=462&height=289)
Converting solar energy by living organisms into hydrocarbon fossil fuels is a complex process. As organisms die, they decompose slowly, usually due to being buried rapidly, and the chemical energy stored within the organisms’ tissues is buried within surrounding geologic materials. All fossil fuels contain carbon that was produced in an ancient environment. In environments rich with organic matter such as swamps, coral reefs, and planktonic blooms, there is a higher potential for fossil fuels to accumulate. Indeed, there is some evidence that over geologic time, organic hydrocarbon fossil fuel material was highly produced globally. [8]. Lack of oxygen and moderate temperatures in the environment seem to enhance the preservation of these organic substances [9; 10]. Also, the heat and pressure applied to organic material after it is buried contribute to transforming it into higher quality materials, such as brown coal to anthracite and oil to gas. Heat and pressure can also cause mobile materials to migrate to conditions suitable for extraction [11].
![By Toby Hudson (Own work) [<a data-cke-saved-href="http://creativecommons.org/licenses/by-sa/3.0" href="http://creativecommons.org/licenses/by-sa/3.0 CC BY-SA 3.0 or GFDL], via Wikimedia Commons The reef has many intricacies.](https://geo.libretexts.org/@api/deki/files/33328/Coral_Outcrop_Flynn_Reef-300x225.jpg?revision=1)
Oil and Gas
Petroleum is principally derived from organic-rich shallow marine sedimentary deposits where the remains of micro-organisms like plankton accumulated in fine grained sediments. [12]. Petroleum's liquid component is commonly called oil and its gas component called natural gas, which is mostly made up of methane (CH4). As rocks such as shale, mudstone, or limestone lithify, increasing pressure and temperature cause the oil and gas to be squeezed out and migrate from the source rock to a different rock unit higher in the rock column. Similar to the discussion of good aquifers, if the rock is sandstone, limestone, or other porous and permeable rock, and involved in a suitable stratigraphic or structural trapping process, then that rock can act as an oil and gas reservoir.

A trap is a combination of a subsurface geologic structure, a porous and permable rock, and an impervious layer that helps block the movement of oil and gas and concentrates it for later human extraction [13; 14]. The development of a trap could be a result of many different geologic situations. Common examples include an anticline or domal structure, an impermeable salt dome, or a fault-bounded stratigraphic block (porous rock next to non-porous rock). The different traps have one thing in common: they pool the fluid fossil fuels into a configuration in which extraction is more likely to be profitable. Oil or gas in strata outside of a trap renders extraction is less viable.

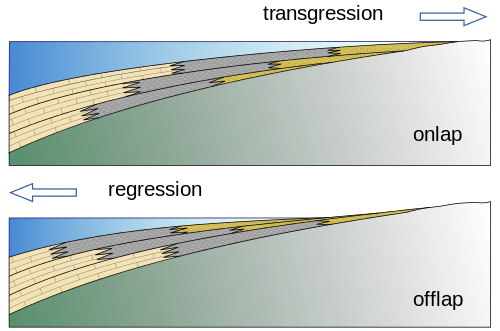
A branch of geology that has grown from the desire to understand how changing sea level creates organic-rich shallow marine muds, carbonates, and sands in close proximity to each other is called sequence stratigraphy [15]. For example, shoreline environments may have beaches, lagoons, reefs, nearshore and offshore deposits, all next to each other. Beach sand, lagoonal and nearshore muds, and coral reef layers accumulate into sediments that include sandstones—good reservoir rocks— next to mudstones, next to limestones, both of which are potential source rocks. As sea level either rises or falls, the location of the shoreline changes and the locations of sands, muds, and reefs with it. This places oil and gas producing rocks (like mudstones and limestones) next to oil and gas reservoirs (sandstones and some limestones). Understanding the interplay of lithology and ocean depth can be very important in finding new petroleum resources because using sequence stratigraphy as a model can allow predictions to be made about the locations of source rocks and reservoirs.
Tar Sands
Conventional oil and gas (pumped from a reservoir) are not the only way to obtain hydrocarbons. The next few sections are known as unconventional petroleum sources, though, they are becoming more important as conventional sources become scarce. Tar sands, or oil sands, are sandstones that contain petroleum products that are highly viscous (like tar), and thus, can not be drilled and pumped out of the ground, unlike conventional oil. The fossil fuel in question is bitumen, which can be pumped as a fluid only at very low rates of recovery and only when heated or mixed with solvents. Thus injections of steam and solvents, or direct mining of the tar sands for later processing can be used to extract the tar from the sands. Alberta, Canada is known to have the largest reserves of tar sands in the world [16].
![By James St. John (jsj1771) http://www.flickr.com/people/jsjgeology/ [<a data-cke-saved-href="http://creativecommons.org/licenses/by/2.0" href="http://creativecommons.org/licenses/by/2.0 CC BY 2.0], via Wikimedia Commons The sandstone is black with tar.](https://geo.libretexts.org/@api/deki/files/33332/Tar_Sandstone_California-300x286.jpg?revision=1&size=bestfit&width=308&height=294)
An energy resource becomes uneconomic once the total cost of extracting and processing it exceeds the revenue which is obtained from the sale of extracted material. Environmental costs may also contribute to a resource becoming uneconomic.
Oil Shale
Oil shale (or tight oil) is a fine-grained sedimentary rock that has a significant quantity of petroleum or natural gas quantities locked tightly in the sediment. Shale is a common source of fossil fuels with high porosity but it has very low permeability. In order to get the oil out, the material has to be mined and heated, which, like with tar sands, is expensive and typically has a negative impact on the environment [17].

Fracking
Another process which is used to extract the oil and gas from shale and other unconventional tight resources is called hydraulic fracturing, better known as fracking [18]. In this method, high-pressure injections of water, sand grains, and added chemicals are pumped underground. Under high pressure, this creates and holds open fractures in the rocks, which aids in the release of the hard-to-access fluids, mostly natural gas. This is more useful in tighter sediments, especially shale, which has a high porosity to store the hydrocarbons but low permeability to transmit the hydrocarbons. Fracking has become controversial due to the potential for groundwater contamination [19] and induced seismicity [20] and its use must balance public and political concerns with energy value.

Coal
Coal is the product of fossilized swamps [21], though some older coal deposits that predate terrestrial plants are presumed to come from algal buildups [22]. It is chiefly carbon, hydrogen, nitrogen, sulfur, and oxygen, with minor amounts of other elements [23]. As this plant material is incorporated into sediments, it undergoes a series of changes due to heat and pressure which concentrates fixed carbon, the combustible portion of the coal. In this sense, the more heat and pressure that coal undergoes, the greater its carbon concentration and fuel value and the more desirable is the coal.
The general sequence of a swamp progressing through the various stages of coal formation and becoming more concentrated in carbon is:
Swamp => Peat => Lignite => Sub-bituminous => Bituminous => Anthracite => Graphite.
As swamp materials collect on the floor of the swamp and are buried under accumulating materials, they first turn to peat. Peat itself is an economic fuel in some locations like the British Isles and Scandinavia. As lithification occurs, peat turns to lignite. With increasing heat and pressure, lignite turns to sub-bituminous coal, bituminous coal, and then, in a process like metamorphism, anthracite. Anthracite is the highest metamorphic grade and most desirable coal since it provides the highest energy output. With even more heat and pressure driving out all the volatiles and leaving pure carbon, anthracite can turn to graphite.

Coal has been used by humans for at least 6000 years [23], mainly as a fuel source. Coal resources in Wales are often cited as a primary reason for the rise of Britain (and later, the United States) in the Industrial Revolution [24; 25; 26]. According to the US Energy Information Administration, the production of coal in the US has decreased due to cheaper prices of competing energy sources and recognition of its negative environmental impacts, including increased very fine-grained particulate matter as an air pollutant, greenhouse gases [27], acid rain [28], and heavy metal pollution [29]. Seen from this point of view, the coal industry as a source of fossil energy is unlikely to revive.
![USGS [Public domain], <a data-cke-saved-href="https://commons.wikimedia.org/wiki/File%3ACoal_anthracite.jpg" href="https://commons.wikimedia.org/wiki/File%3ACoal_anthracite.jpg via Wikimedia Commons It is very black and shiny.](https://geo.libretexts.org/@api/deki/files/33329/Coal_anthracite-300x281.jpg?revision=1)
As the world transitions away from fossil fuels including coal, and manufacturing seeks strong, flexible, and lighter materials than steel including carbon fiber for many applications, current research is exploring coal as a source of this carbon.
References
9. Gordon, M., Jr, Tracey, J. I., Jr & Ellis, M. W. Geology of the Arkansas bauxite region. (1958).
12. Pratt, W. E. Oil in the Earth. (University of Kansas Press, 1942).
14. Dott, R. H. & Reynolds, M. J. Sourcebook for petroleum geology. (1969).
16. Bauquis, P.-R. What future for extra heavy oil and bitumen: the Orinoco case. in 13, 18 (1998).
17. Youngquist, W. Shale oil--The elusive energy. Hubbert Center Newsletter 4, (1998).
24. Belloc, H. The Servile State. (T.N. Foulis, 1913).
25. McKenzie, H. & Moore, B. Social Origins of Dictatorship and Democracy. (1970).


