1.6: Essential Chemistry and Physics Concepts for Oceanography
- Page ID
- 9694
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Aspects of chemistry and physics are discussed in nearly every chapter on oceanography. Below are highlights of important concepts.
What is Matter?
Basic concepts of chemistry are essential to understanding the physical and chemical properties of matter, particularly natural earth materials (rocks, seawater, air, organic matter, etc.). The chemical characteristics of earth materials reveal information about the environments how and where they are formed, Their characteristics also determine their potential fate when exposed to chemical changes over time. For instance, rocks formed deep underground may not be stable in the surface environment where they are exposed to water, air, temperature changes, and other physical and chemical conditions.
Basic chemistry concepts needed to be understood for this oceanography course include:
- All matter is made up of atoms, and atoms are made up of atomic particles (electrons, protons, and neutrons). An atom is the smallest unit of a chemical element. Atoms have a nucleus composed of neutrons & protons and has a positive charge. Negatively charged electrons orbit around the nucleus in shell-like layers.
- A chemical element is a pure chemical substance consisting of one type of atom distinguished by its atomic number, which is the number of protons in its nucleus. Elements have equal balance in numbers of positively charged protons and negatively charged electrons. Common examples of elements are iron, copper, silver, gold, hydrogen, carbon, nitrogen, and oxygen.
- An element is a substance that cannot be broken down into simpler substances by chemical means.
- An element is composed of atoms that have the same atomic number, that is, each atom has the same number of protons in its nucleus as all other atoms of that element.
- The Periodic Table is a list of known chemical elements arranged in order from smallest to largest and by group chemical properties. It is a list of 118 known elements arrange by atomic number. Of these, 92 are naturally occurring (prior to development of artificial nuclear research and development; elements 95 to 118 have only been artificially created and are highly unstable). The lightest element, hydrogen, has one proton, whereas the heaviest naturally occurring element, uranium, has 92 protons. In general elements on the left side of the periodic table are metals, and elements on the right (shown in blue in Figure 1.15) are nonmetals.
- Atoms bond together to form molecules. A molecule is a group of atoms bonded together, representing the smallest fundamental unit of a chemical compound that can take part in a chemical reaction.
- A chemical compound is a pure chemical substance consisting of two or more different chemical elements that can be separated into simpler substances by chemical reactions. Chemical compounds have a unique and defined chemical structure; they consist of a fixed ratio of atoms that are held together in a defined spatial arrangement by chemical bonds. All minerals are chemical compounds, but by comparison relatively few compounds are naturally occurring minerals!
- Types of molecular bonds include metallic (for metals), ionic (compounds that dissolve easily), covalent (most others).
- A mixture is a combination of two or more pure substances in which each pure substance retains its individual chemical properties. Examples of mixtures include rocks, magma (molten rock) air, and seawater.
- Chemical formulas are used to describe compounds such as H2O (for water), NaCl (for salt), CO2 (for carbon dioxide)
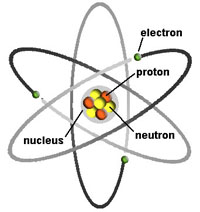
Fig. 1-18. Structure of an atom: this example is the element lithium composed of a nucleus of 3 protons, 4 neutrons, and an outer shell of 3 electrons spinning around the nucleus.
The most abundant elements in our physical environment are: H, C, N, O, Na, Mg, Al, Si, P, S, Cl, K, Ca, Fe (Be prepared to name these elemental symbols! -- see Figure 1.15).
These elements are:
- ingredients of common rocks and sediments (solids)
- components of seawater and air (liquids & gases)
- essential nutrients for life (organic compounds)

Fig 1-19. The periodic table with essential elements highlighted.
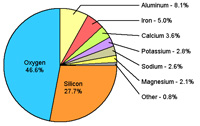
Figure 1.20. Composition of the crust. Rock samples collected from around the world show that the chemical composition of the Earth's crust is not uniform, but certain elements are much more abundant than others. Silicon and oxygen are the two most abundant elements in the crust.


