7.2: The Producers
- Page ID
- 4507
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Although the phytoplankton are microscopic in size compared to marine plants and macroalgae like seaweeds and seagrasses, they account for by far the greatest amount of photosynthesis in the oceans; about 95% of all marine primary productivity. Most of the production by phytoplankton comes from three groups, the diatoms, dinoflagellates, and coccolithophores.
Diatoms are single-celled algae consisting of cellular material inside a shell, or test, made of silica, a component of glass. Diatoms are relatively large, reaching up to about 1 mm in diameter, and come in a range of shapes, from circular disks to elongated or triangular forms (Figure \(\PageIndex{1}\)). In some species of diatoms individual cells link together into multicellular chains. Diatoms are very efficient producers, with up to 55% of the absorbed sunlight energy incorporated into carbohydrate formation; this is one of the highest photosynthetic efficiencies known. Diatoms are most abundant in coastal and cold, nutrient-rich waters. Where diatoms are bountiful, the underlying sediments are rich in their silica shells, creating siliceous sediment and diatomaceous earth (see section 12.3).
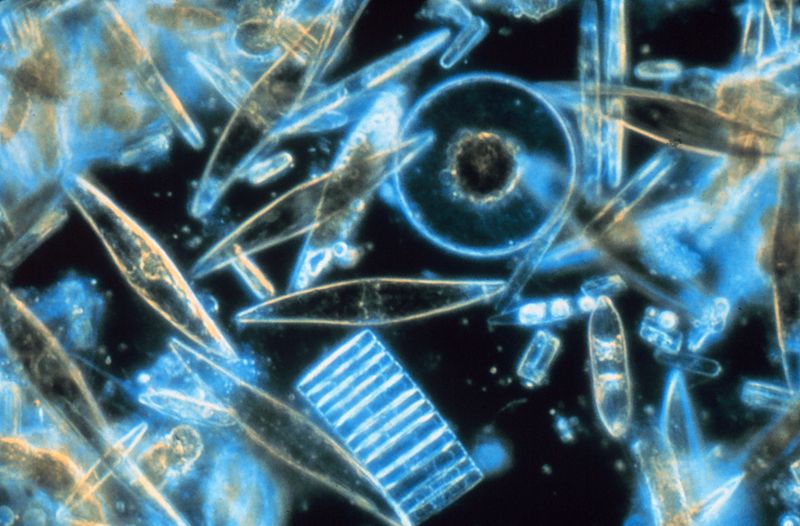
Dinoflagellates are another form of single-shelled photosynthetic algae that are generally smaller than diatoms, most with sizes in the 0.015 – 0.04 mm range. Most dinoflagellates have a characteristic pair of flagella (hence the name); small whip-like “tails” that they use for locomotion. Usually there is one flagellum that trails from the body to provide forward movement, and another that encircles the cell to make it spin as it moves. Unlike diatoms, dinoflagellates do not have a mineralized shell. Instead, many are covered by cellulose, which easily decomposes in seawater, so their shells do not really contribute to sediment formation (Figure \(\PageIndex{2}\)). While most dinoflagellates undergo photosynthesis, some species will also ingest prey.
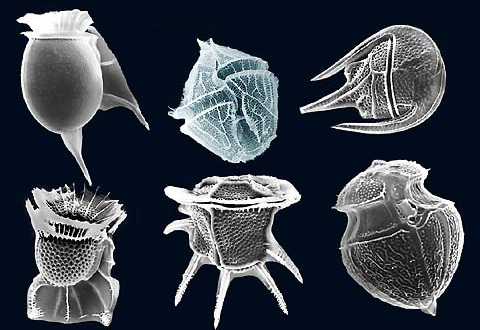
A third, much smaller type of phytoplankton includes the coccolithophores, which range from about 5-100 micrometers wide. As with diatoms and dinoflagellates, these are single-celled photosynthetic algae that contribute significantly to oceanic primary production, but their cellular material is encased in a very different kind of shell. The test (shell) is made up of a number of interlocking circular plates composed of calcium carbonate that link together to form a sphere (Figure \(\PageIndex{3}\)). Coccolithophores are most abundant in warm, open ocean waters, and their sinking tests can lead to calcium carbonate sediments in some parts of the ocean (see section 12.3).

Recent evidence suggests that another group of organisms, the bacteria, or picoplankton, may be very important primary producers. Although they are very small, on the order of 0.2-2 micrometers long, they can be found in very high concentrations, and may be responsible for up to 70% of all productivity in some parts of the ocean.
Harmful algal blooms
Primary production provides plentiful food resources for ocean consumers, so a high abundance of phytoplankton is a good thing, right? As in many other cases, too much of a good thing can sometimes be dangerous, and an overabundance of dinoflagellates or diatoms can often create serious concerns. These events are referred to as harmful algal blooms, or HABs. HABs can occur for a number of reasons, although a common one is an overabundance of nutrients, which is often due to excessive terrestrial runoff of fertilizers or other nitrogen- and phosphate-containing materials. These conditions lead to an explosion in algal populations that can change the color of the water if the cells are in high enough concentrations. Figure \(\PageIndex{4}\) shows a massive bloom that contained so many dinoflagellate cells that it turned the water reddish-brown, a so-called “red tide.” (It has been suggested that Biblical references to seas being “turned to blood” may have actually been describing red tide events).

These massive algal blooms can have some serious consequences. For one, when all of this algae eventually dies off and sinks, their decomposition uses up the dissolved oxygen in the water, leaving anoxic or hypoxic conditions that can lead to the mass die-off of fish and invertebrates. Dinoflagellates and diatoms are also capable of producing toxins themselves. These phytoplankton get eaten by fish, shellfish and other organisms, and in high abundances the toxins become concentrated in the tissues of the consumers. When humans or other higher-level consumers then eat these organisms, the toxins are concentrated enough to cause sickness or even death. For example, some dinoflagellates produce a toxin that causes paralytic shellfish poisoning, which can occur in humans as soon as 30 minutes after eating infected shellfish. This toxin attacks the nervous system, producing symptoms of dizziness, nausea, slurred speech, loss of feeling, and uncoordinated movements, and can ultimately be fatal. Diatoms produce a toxin called domoic acid that causes amnesic shellfish poisoning, leading to memory loss, seizures, and potentially death. Domoic acid poisoning also affects marine animals; it is thought to have been responsible for an event in Capitola, California in 1961 where flocks of seabirds acted crazily, even attacking humans. This event inspired the Alfred Hitchcock movie “The Birds.”
Additional links for more information:
- For more on harmful algal blooms, visit NOAA’s HAB site: http://www.noaa.gov/what-is-harmful-algal-bloom


