5.4: Oxygen
- Page ID
- 4497
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Ions are not the only materials that are dissolved in seawater. The oceans also contain dissolved gases that are very important to living organisms, particularly oxygen (O2), carbon dioxide (CO2), and nitrogen (N2). Oxygen is required for respiration in marine plants, algae, and phytoplankton (the primary producers) and animals. Carbon dioxide is utilized by the primary producers to power photosynthesis, a byproduct of which is oxygen. Nitrogen gas dissolved in the ocean is fixed by bacteria and converted into the forms required for primary production, such as nitrate and nitrite.
All of these gases are found in the atmosphere, and can enter the ocean by dissolving into the water at the ocean’s surface. But the amount of each gas in air is very different from the amount found in the ocean (Table 5.4.1).
[table id=6 /]
The amount of each gas that can dissolve in the ocean depends on the solubility and saturation of the gas in water. Solubility refers to the amount of a dissolved gas that the water can hold under a particular set of conditions, which are usually defined as 0o C and 1 atmosphere of pressure. The solubility of a gas increases with increasing pressure, decreased temperature, and decreased salinity. Saturation refers to the amount of gas currently dissolved in the water, relative to the maximum possible content. If the water is undersaturated, more gas can dissolve. If the water is saturated or supersaturated, gas may be released. Most atmospheric gases are saturated in the ocean, but O2 and CO2 are not saturated because they are rapidly used by living organisms.
Oxygen
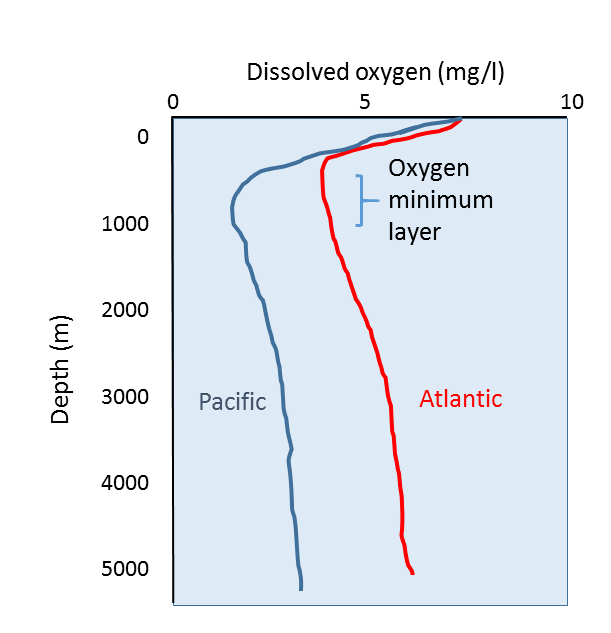
Typical oceanic dissolved oxygen profiles are shown in Figure \(\PageIndex{1}\). The shape of the profile is determined by the various processes that add or remove oxygen from the water at different depths.
Oxygen content is highest at the surface for two main reasons; this is where oxygen dissolves into the ocean from the atmosphere, and the surface water is where oxygen is produced by phytoplankton through photosynthesis. Respiration is also occurring in the surface waters, but the rate of photosynthetic oxygen production is greater than the rate of removal through respiration. It should be noted that even though dissolved oxygen is highest at the surface, there is still far less oxygen in the water than is found in the air. Well-oxygenated surface water may only contain around 8 mg O2/l, while the air contains 210 mg O2/l.
As depth increases, dissolved oxygen declines, reaching a minimum between a few hundred meters and 1000 m deep, the aptly-named oxygen minimum layer. At these depths and below, the water is too far removed from the surface for any atmospheric exchange, and there is not enough light to support photosynthesis, so there is little if any oxygen added to the water. At the same time, oxygen is removed from the water through the respiration of deep water organisms, and the decomposition of organic material by bacteria as it sinks to depth.
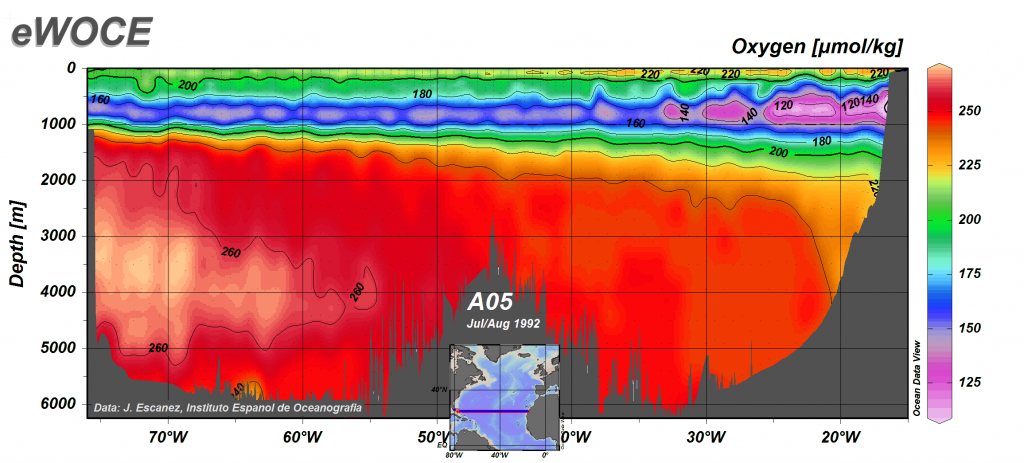
Below the oxygen minimum layer there is often an increase in dissolved oxygen at the greatest depths (Figures 5.4.1, 5.4.2). This bottom water is usually colder than the surface water and is under enormous pressure; as stated above, lower temperatures and higher pressure increase the solubility of dissolved gases. But there is another reason that bottom water contains more oxygen than mid-water depths that has to do with the way water circulates throughout the deep ocean (see section 9.8). In polar regions, the cold surface water absorbs lots of oxygen. This cold, oxygen-rich water sinks to the bottom due to its high density, taking the oxygen with it. The oxygen-rich bottom water will then spend the next thousand years or so moving over the seafloor throughout the major ocean basins. This deep water circulation is the source of oxygen for bottom-dwelling (benthic) organisms. The oxygen-rich bottom water forms in the polar regions of the Atlantic, and slowly makes its way to the Pacific, with oxygen being removed for respiration along the way. This is why dissolved oxygen levels in Pacific deep water are generally lower than in the Atlantic (Figure \(\PageIndex{1}\)).
Areas where dissolved oxygen levels are too low to support most life are referred to as hypoxic zones (they are experiencing hypoxia, or low oxygen). Hypoxia is usually defined as oxygen levels below 2 mg/L. Anoxic zones (anoxia = without oxygen) show more severe forms of hypoxia, with oxygen below 0.5 mg/L. Some parts of the oceans may experience seasonal or temporary periods of hypoxia, while in other areas these conditions may last much longer. These hypoxic conditions often lead to mass die-offs of marine organisms who struggle to survive without sufficient oxygen.
Additional links for more information:
- NOAA site on oceanic hypoxic zones: http://oceanservice.noaa.gov/hazards/hypoxia/


