7.7: Another Big Whammy for Coral Reefs: Increasing Atmospheric Carbon Dioxide?
- Page ID
- 14969
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)
Figure 7-1: a shot of part of the HUB aquarium at Penn State in which there is a living, thriving coral "reef". Come visit!
Yes, even at landlocked Penn State, we have a piece of shallow-marine environments, right in the midst of the bustle of the Hetzel Union Building (HUB) on campus. Thanks to the class of '99, a dedicated faculty member from Chemistry and a string of dedicated students this aquarium and its denizens continue to thrive. Here (link is external)is a brief article about the aquarium (2002) with pictures. One of the things learned in maintaining such small ecosystems is the need to buffer changes in certain chemical substrates, particularly dissolved carbon, pH and Calcium ions, all of which are essential to allowing corals to precipitate their aragonite (CaCO3) skeletons. The aquarium maintains a bed of granulated limestone through which fluids are circulated before they enter the tank. This allows some of the limestone to dissolve, if needed, contributing the essential Ca2+ and CO3-2 ions for incorporation by growing, skeletonizing corals. The chemical reaction would be:
Ca2+ + CO32- ----> CaCO3(s) (aragonite) (this reaction is, of course governed by a temperature-sensitive equilibrium "constant")
As you learned in the marine chemistry lesson, solutions must be at or above saturation with respect to a given mineral to allow it to precipitate. Inadvertently, however, we are beginning to lower the level of saturation of the surface ocean with respect to aragonite, and this will make it more difficult for corals and other aragonitic organisms to precipitate shells or skeletons. How does this work?
First, you have probably seen various versions of Figure 7-2.
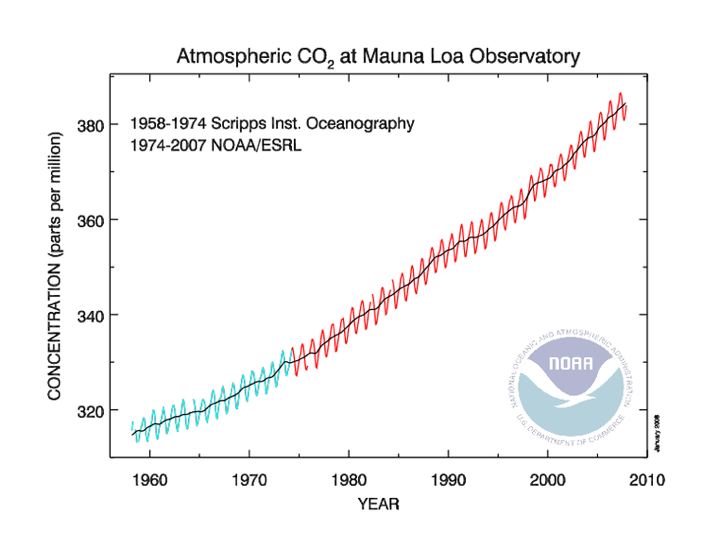
Figure 7-2: Concentration of carbon dioxide in the atmosphere (parts per million; ppm) from 1958-2008 from monitoring atop Mauna Loa on Hawaii.
Source: NOAA
This record illustrates an increase in the average (the little wiggles are seasonal variations) pCO2 (partial pressure of carbon dioxide in the atmosphere) of about 19 percent over the past half century. Of course, it is this increase that global warming has been attributed to, but there is another issue. Even if we could mitigate global warming by some engineering miracle (mirrors in space, etc.), the increase in pCO2 would probably ultimately get the corals. Why? Because the increase in pCO2 and its penetration into the ocean surface lowers the pH of the ocean, decreasing the carbonate ison concentration and, ultimately, decreasing saturation with respect to aragonite. Figure 7-3 shows how this works with some simple calculations by doubling pCO2.
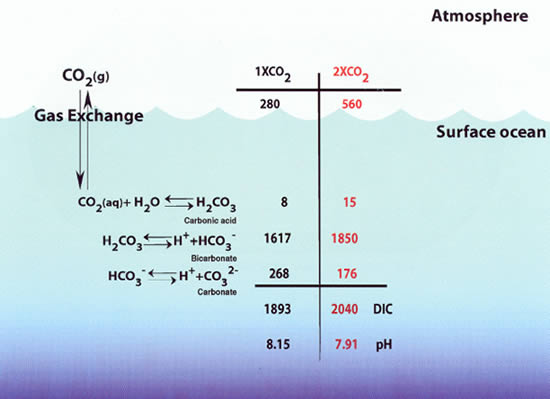
Figure 7-3
Source: NOAA/PMEL
Note that through reactions shown (carbonic acid equilibria in seawater) doubling pCO2 from its pre-industrial level (280 ppm) to 560 ppm substantially decreases pH (by 0.24 units) and carbonate ion concentration (by about 34 %); of course, we have not gotten to that point--yet! Note, however, that atmospheric CO2 derived from fossil fuels (how do we know this?) has mixed down into surface waters and penetrated deeper into the ocean (remember the deep circulation?) in some regions as shown in Figure 7-4. This is causing a decrease in pH.
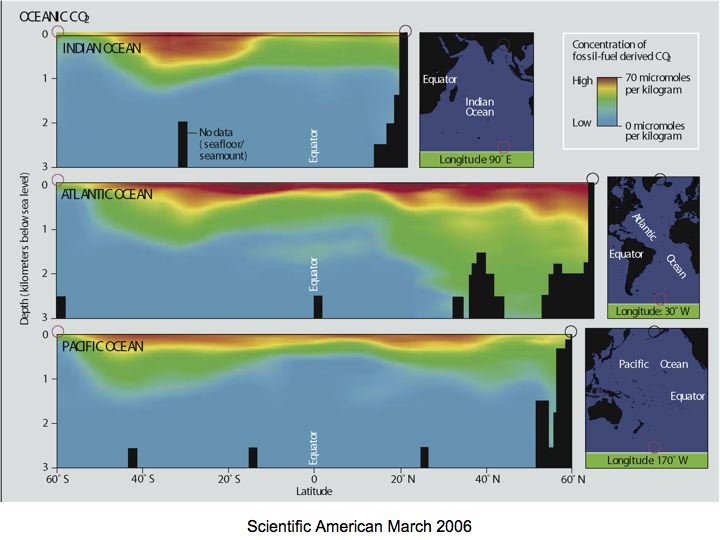
Figure 7-4
Source: Scientific American, 2006
Chris Langdon (Lamont Doherty Earth Observatory, Columbia University) grew corals artificially in Biosphere 2, Arizona and subjected them to different pCO2 levels. He found that their growth rate decreased with increasing pCO2 as shown in Figure 7-5.
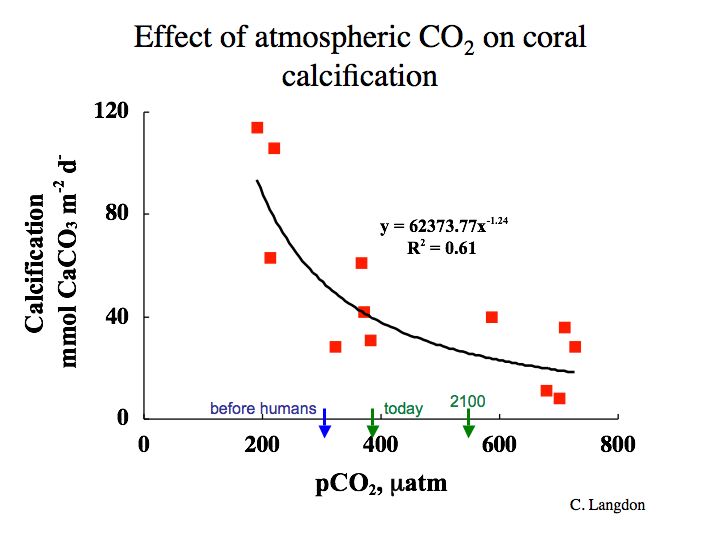
Figure 7-5: calcification rate (precipitation of aragonite) of corals in seawater at constant temperature as a function of atmospheric pCO2 in Biosphere 2 (closed system) experiments performed by Chris Langdon (LDEO). Note decreasing rate of calcification and benchmarks for the future. It looks as though the corals may already be having trouble, at least relative to the pre-industrial world. What do you think?


