4.4.6: Crystal Twinning
- Page ID
- 19160
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)

In ideal crystals, atoms are in repetitive arrangements, oriented the same way in all parts of the crystal. Twinning result when different domains of a crystal have different atomic orientations. The photo of twinned gypsum on the left (Figure 4.39) is a good example. Half the crystal grew with atoms oriented differently from atoms in the other half. This kind of twinning of gypsum is called swallowtail twinning, for obvious reasons.
Some twinning, called contact twinning, appears as two or more crystal domains in contact with each other (like the gypsum above). The domains share atoms along a common surface, typically a plane called the composition plane. Twins differ from crystal intergrowths composed of crystals that grow next to each other. In a twinned crystal the structure and bonds continue across the composition plane; in intergrowths they are discontinuous.
Another kind of twinning, called penetration twinning, appears as crystals that seem to have grown through each other. In such twins, two domains share a volume of atoms, not just a plane of atoms. The twinned staurolites in Figure 4.40 are good examples of penetration twins. The staurolite specimen includes both a cruciform (cross-like) twin, sometimes called a fairy-cross, and a V-twin that resemble slightly the twinned gypsum on the left. The fluorite crystals in Figure 4.37 also display penetration twins.

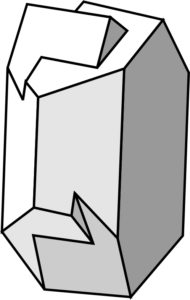
Simple twins comprise only two domains that share common planes or volumes of atoms. The gypsum and staurolite seen above are examples. The twinned orthoclase (K-feldspar) crystals seen here in Figure 4.41 are also examples of simple penetration twinning. The drawing better depicts the nature of the crystal intergrowths.
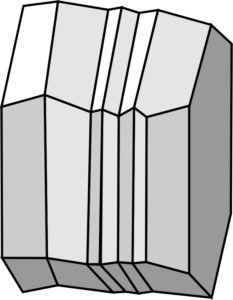

Crystals with complex twins, in contrast with simple twins, have more than two individual twin domains. The drawing and photo (Figures 4.43 and 4.44) show complex twinning called polysynthetic twinning that often characterizes plagioclase, calcite, and a few other minerals. The mineral specimen shows twin striations (stripes) with different reflectivities, because the alternating domains contain atoms arranged in slightly different ways. The presence of striations is one way that geologists distinguish plagioclase from other feldspars, such as the orthoclase seen in Figure 4.41.

Figure 4.45 shows another example of complex twinning called cyclic twinning. The mineral is cerussite (PbCO3). In cyclic twins, three or more crystals seem to emanate from a central point, so the different crystal domains are not parallel but instead are radiating. This photo shows one very important feature. Identifying twins in hand specimens can be difficult, especially in poorly formed crystals. One diagnostic characteristic is the presence of reentrant angles, like the ones seen in this twinned cerrusite. Two crystal faces intersect to form a reentrant angle when they produce an angular concavity that points toward the interior of a crystal instead of the (normal) exterior.
Twinning comes at all scales and may be difficult to detect. Sometimes we can see it with the naked eye, sometimes we can only see it with a microscope, and sometimes we cannot detect it without more sophisticated devices.
Whether twinning is simple or complex, atoms in different twin domains are related by some kind of twin symmetry. For example, the atomic arrangements in two domains may be mirror images of each other. This is the case for all the fine striations in the plagioclase crystal in Figure 4.44 – the alternating domains are reflections of each other. If not mirror images, two twin domains may be related by rotation, such as in Figure 4.45, and there are other ways that domains can be related, too. So, there are many kinds of twins. The nature of a particular kind – whether it is simple or complex and the kind of symmetry involved – define what is called a twin law and allow different kinds of twins to be named. For example, the plagioclase twinning in Figure 4.44 is albite twinning, and the K-feldspar twinning in figure 4.41 is Carlsbad twinning. The domains in albite twinning are related by reflections across a near vertical plane. The two domains in Carlsbad twins are related by a rotation around a near vertical axis in the crystals shown.
Twins form in several ways. They may be growth twins, transformation twins, or deformation twins. Growth twins form when a crystal first grows. Atoms being added to the outside of an already existing crystal may become slightly misplaced so that all subsequent atoms are arranged in a different orientation than in the original crystal domain. Essentially, a new crystal (a new domain) develops adjacent to the original. If a common plane of atoms is oriented correctly for both domains, the result is a contact twin. If a common volume of atoms is oriented correctly for both domains, a penetration twin has been formed. These relationships are most easily visualized by looking at some of the photos and drawings above.
Transformation twins may form when an existing mineral goes through a phase transition to become a different mineral. This involves polymorphs. For example, the feldspar sanidine (KAlSi3O8) forms at high temperature in volcanic rocks. With cooling it may change into orthoclase, a different form of KAlSi3O8, and then microcline, a third form of KAlSi3O8. During the transformations, atoms in different domains of the crystal may become slightly misoriented with respect to other domains. So, twinned orthoclase and microcline crystals may be the result.
The third kind of twins, deformation twins, may develop if a crystal is subjected to stress. Planes or volumes of atoms may become slightly displace, producing domains with different orientations. This kind of twinning is common in calcite, although generally a microscope is needed to see it. Deformation twins are generally not of great importance to mineralogists.


