4.4.4: Crystal Defects
- Page ID
- 19140

A hypothetical perfect crystal has an ordered atomic structure with all atoms in the correct places. Yet, as pointed out by C. G. Darwin in 1914, such crystals cannot exist. While a crystal may look perfect on the outside, atomic structures always contain some flaws, called defects.
Today, techniques involving X-ray, transmission electron microscope (TEM), and, most recently, high-resolution transmission electron microscope (HRTM) allow crystallographers to look at atomic arrangements and to see relationships between individual atoms. The image in Figure 4.34 shows the atomic structure of crocidolite, an asbestiform amphibole, obtained with a transmission electron microscope. The black and white colors are atomic units composed of a small number of atoms. The entire view shows an imperfect grain composed of multiple subgrains with slightly different atomic orientations (shown by the letters and vectors labeling crystallographic axes – discussed in a later chapter). The apparent offsets in the structure, called zipper faults, are lines along which the atomic structure is defective. In some places, especially along subgrain boundaries, a coarsening of texture suggests small areas that have atomic structure dissimilar from that of normal crocidolite.
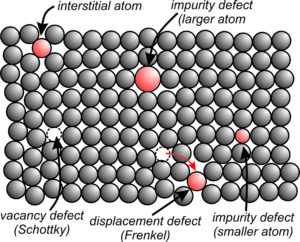
Perhaps the simplest type of defect is an impurity defect, occurring when a foreign atom is present in a mineral’s atomic structure (Figure 4.35). No mineral is perfectly pure. Minerals always contain minor or trace amounts of elements not described by their formula, often at levels that we cannot detect using standard analytical techniques. As seen in this schematic drawing, a larger or smaller atom may replace one normally in the structure, or an atom may occupy an interstitial site. All these examples are types of point defects, so named because they occur at one or a few points in the structure.
Other types of point defects include Schottky and Frenkel defects (both shown in the Figure 4.35. Schottky defects occur when an atom is displaced from a structure altogether, leaving a vacancy or hole. Such defects involve both cations and anions and, to maintain charge balance, missing anions must be matched by missing cations. Frenkel defects occur when an atom is displaced from the position it normally occupies to an interstitial site. Frenkel defects affect both cations and anions, but cation defects are more common because anions are larger and usually more tightly bonded in place.
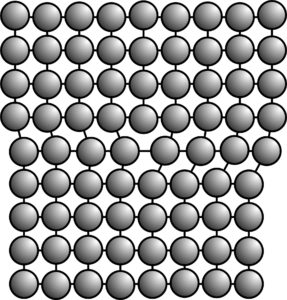
Besides point defects, other types of defects include line defects and plane defects. Line defects, including edge dislocations, like the one shown in the schematic in Figure 4.36, are defects that end at lines in a structure. Plane defects, as their name implies, are planes along which a crystal’s structure is displaced or distorted. On a large scale, grain boundaries are types of plane defects. At the atomic level, several different structures may separate slightly misoriented portions of a crystal structure so that a crystal contains domains having slightly different atomic orientation. Domains of this sort are clearly seen in the TEM photograph above (Figure 4.34).


