4.2.1: Igneous Minerals
- Page ID
- 19132
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)In magma (molten rock), kinetic energy means that atoms are always in motion. The photo in Figure 4.8 shows lava (magma that has reached Earth’s surface) in Hawaii. When magma is at high temperature, it is completely liquid because high kinetic energy ensures that no solid is stable. Some atoms collide and may form bonds temporarily before breaking apart again. A balance exists between the formation of bonds and the rate at which they break apart. If bonds break as fast as they form there will be no net crystallization.


As magma cools, kinetic energy decreases when atoms slow down. Eventually, if magma cools sufficiently, atoms will slow down enough so that some bonds will begin to persist. This is the beginning of the formation of crystals from a melt, and the beginning of the formation of igneous minerals. Initial crystallization creates small nuclei, many of which continue as the centers of crystals during continued growth. Because of high temperatures and the molten state of magma, atoms are quite mobile and easily move toward the nuclei and to surfaces of growing crystals. So crystals may become large, like the dark colored pyroxene and light grey plagioclase in the gabbro in Figure 4.9.
The table below lists some common minerals in igneous rocks. Almost all are silicates because the magmas that produce igneous rocks are dominated by oxygen and silicon. Other abundant elements include aluminum, iron, magnesium, calcium, sodium, and potassium. These elements make up most igneous minerals.
| Common Minerals in Igneous Rocks | ||||
| class or group | minerals or series | chemical formula | ||
| olivine | olivine | (Mg,Fe)2SiO4 | ||
| pyroxene | diopside augite orthopyroxene |
CaMgSi2O6 (Ca,Mg,Fe,Na)(Mg,Fe,Al)(Si,Al)2O6 (Mg,Fe)2SiO6 |
||
| amphibole | hornblende | (K,Na)0-1(Ca,Na,Fe,Mg)2(Mg,Fe,Al)5(Si,Al)8O22(OH)2 | ||
| mica | biotite muscovite |
K(Mg,Fe)3(AlSi3O10)(OH)2 KAl2(AlSi3O10)(OH)2 |
||
| feldspar | orthoclase microcline sanidine plagioclase |
KAlSi3O8 KAlSi3O8 KAlSi3O8 (Ca,Na)(Si,Al)4O8 |
||
| feldspathoid | leucite nepheline |
KAlSi2O6 (Na,K)AlSiO4 |
||
| silica | quartz | SiO2 | ||
| oxide | magnetite ilmenite |
Fe3O4 FeTiO3 |
||
| sulfide | pyrite pyrrhotite |
FeS2 Fe1-xS |
||
| other | titanite zircon apatite |
CaTiSiO5 ZrSiO4 Ca5(PO4)3(OH,F,Cl) |
||
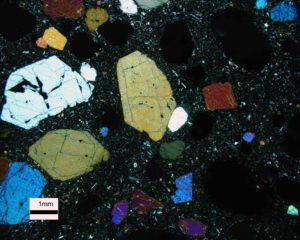
If the conditions are right, igneous mineral crystals may develop prominent crystal faces. The photo here in Figure 4.10 is a microscope view of Hawaiian basalt. It was obtained using a petrographic microscope. The largest gray, yellow, and blue minerals are pyroxene, but their colors, called interference colors, are not the true colors of the mineral. Somewhat smaller minerals with brighter colors are olivine. The pyroxene shows cleavages and the olivine does not. Because they had room to crystallize and grow from a magma, some of the olivine and pyroxene crystals have (imperfectly formed) crystal faces. The black material with fine white flecks in the background is basaltic glass containing fine crystals of plagioclase.
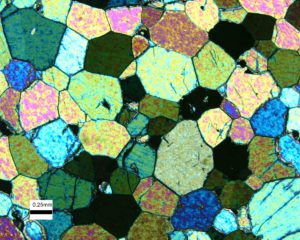
If crystals do not have room to grow individually, and instead crystallize simultaneously, the result may be a rock with crystals forming a mosaic pattern, like the rock seen in Figure 4.11. This view shows grains of olivine in a dunite (a rock composed nearly entirely of olivine). The colors, like the colors in the previous figure, are not true mineral colors but are artifacts of the way this rock was viewed. Olivine, pyroxenes, feldspars, and many other minerals commonly develop this kind of texture.
Igneous processes are quite variable. Some magmas cool slowly underground, so mineral crystals grow to be large. Other magmas extrude as lavas and cool quickly to form basalt or another extrusive rock. Mineral crystals in extrusive rocks may be so small that they cannot be seen with the naked eye or even with a microscope. Silicate minerals dominate igneous rocks but magma compositions vary somewhat. So igneous rocks have variable compositions and consequently variable mineralogies.
4.2.1.1 Pegmatites

Pegmatites are exceptionally coarse grained igneous rocks. In some pegmatites, crystals may be huge. The photo seen here shows white quartz, salmon-colored feldspar and black riebeckite in a pegmatite. The hand lens is about 2 cm across. Pegmatites form during the final stages of magma crystallization. Most have overall composition similar to that of granite.
The largest crystals in the world have been found in pegmatites. A single crystal of mica (phlogopite) from Ontario, Canada, is 4.2 m (14 ft) wide and 10 m (33 ft) long. A quartz crystal from a Russian pegmatite weighs more than 907 kg (2,000 lbs.). The largest quartz crystal on record, however, was from a pegmatite in Brazil and weighed more than five tons.

Some pegmatites are enriched in elements that are normally minor components of magmas. The photo seen here is a crystal of watermelon tourmaline from a pegmatite. Tourmaline is really the only common mineral that contains boron.
Other elements that concentrate in pegmatites include cesium, beryllium, zirconium, niobium, uranium, thorium, tantalum, tin, rare earth elements, chlorine, fluorine, lithium, and phosphorus. Consequently, pegmatites are sometimes mined for these elements. Pegmatites also yield gemstones, including varieties of tourmaline like the one shown, bright green feldspar called amazonite, several varieties of beryl (emerald, aquamarine, and heliodor), and others.


