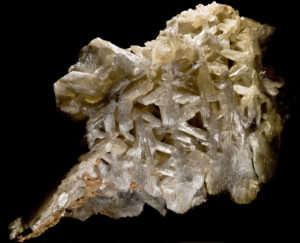
3.3.2: Mineral Habit
- Page ID
- 19162
The photos above (Figure 3.16) show examples of different mineral habits. Habit, a property closely related to crystal shape, includes shape and size of crystal faces, how forms combine, how well developed different forms are, and the way multiple crystals grow together. Habit, thus, is the characteristic appearance a mineral can have. The photos in Figure 3.16 show some contrasting examples.
The most useful terms describing habit are self-explanatory. Common ones used to describe the habit of single crystals include equant (equidimensional), acicular (needlelike), tabular, and bladed. These and other terms are defined below.
| Terms Used to Describe Shapes of Individual Crystals (With Example Minerals) | ||
| equant | the same dimensions in all directions (garnet, spinel) | |
| blocky | equant with a nearly square cross sections (halite, galena) | |
| acicular | needlelike (actinolite, sillimanite) | |
| tabular or platy | appearing to be a plates or a thick sheet (gypsum, graphite) | |
| capillary or filiform | hairlike or threadlike (serpentine, millerite) | |
| bladed | elongated and flattened in one direction (kyanite, wollastonite) | |
| prismatic or columnar | elongated with faces parallel to a common direction (apatite, beryl) | |
| foliated or micaceous | easily split into sheets (muscovite, biotite) | |
For describing an assembly of multiple crystals, we use terms such as massive, granular, radiating, and fibrous. We list and define these terms and others in this table:
| Terms Used to Describe Properties of Crystal Aggregates | |
| massive | appearing as a solid mass with no distinguishing features |
| granular | composed of many individual grains |
| radiating or divergent | containing crystals emanating from a common point |
| fibrous | composed of fibers |
| stalactitic | appearing stalactite shaped |
| lamellar or tabular | appearing like flat plates or slabs growing together |
| stellated | containing an aggregate of crystals giving a starlike appearance |
| plumose | having feathery appearance |
| arborescent or dendritic | appearing like a branching tree or plant |
| reticulated or latticelike | net-like, composed of slender crystals forming a lattice pattern |
| colloform or globular | made of spherical/hemispherical shapes made of radiating crystals |
| botryoidal | having an appearance similar to a bunch of grapes |
| reniform | having a kidney-shaped appearance |
| mammillary | having breastlike shape |
| drusy | having surfaces covered with fine crystals |
| elliptic or pisolitic | composed of very small or small spheres |
Unfortunately, although museum specimens and pictures of minerals in textbooks often show distinctive shapes and habits, most mineral samples do not. Small anhedral crystals without flat faces, or massive aggregates of many small crystals, are typical, sometimes rendering shape and habit of little use for identification. Additional complications arise because some minerals, for example calcite, have different crystal shapes or habits, depending on how they grow. Nonetheless, shape and habit reflect the internal arrangement of atoms in a crystal and, when visible, can be important diagnostic properties. For example, the movie makers blew it in the 1995 movie Congo.
Box 3-1 What’s Wrong With This Picture?

In the 1995 movie Congo, an exploration team goes to Africa to seek large, flawless diamonds. When the diamonds are shown, the movie immediately loses credibility with mineralogists because the crystals are hexagonal prisms (long crystals with a hexagonal cross section). Mineralogists know that diamond crystals can never be hexagonal prisms. The photo shows actor Timothy Curry holding a quartz crystal, not a diamond crystal.
For additional views of many different mineral habits with a discussion, go the video linked below. It does a good job of defining many of the terms used to describe different habits that are listed in the tables above:
Video 3-2: Examples of mineral habits (10 minutes)
Asbestos mineral have, for a long time, been known for posing health risks because of their habits. The box below discusses these minerals and risks.
Box 3-2 Asbestiform Minerals and Health Risks

We use the term asbestiform to describe a mineral habit characterized by small, strong, and flexible fibers, equivalent to hairs or whiskers. Asbestos is a commercial name for any marketable asbestiform mineral. For legal and regulatory purposes, however, the US Environmental Protection Agency has developed a more restrictive definition and defines asbestos as being fibrous varieties of one of six specific minerals. Other countries have similar legal definitions.
Mineralogists have described many asbestiform mineral varieties, but most are rare and only a few are produced for sale. The photo seen in Figure 3.20 shows “white asbestos,” composed of the mineral chrysotile. Chrysotile, which accounts for about 95% of the commercial asbestos market, is a member of the serpentine mineral group. It is a widespread but minor mineral in many altered ultramafic rocks.
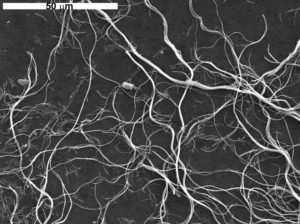
Some commercial asbestos is composed of crocidolite (“blue asbestos”) or amosite (“brown asbestos”), varieties of the amphiboles riebeckite and grunerite, respectively. Other minerals that may be asbestiform include other amphiboles (anthophyllite, tremolite, actinolite), clays (sepiolite, palygorskite), and some members of the zeolite mineral group.
Historically, asbestos has had many uses. Since around 1880, it has been mined in large quantities because it is tough, flexible, and fire and chemical resistant. Between 1900 and 1986, builders sprayed asbestos on walls, ceilings, and pipes in many buildings in the United States. Industries have used asbestos in brake linings, roof shingles, and other applications. Unfortunately, asbestos easily crumbles to make a fine dust that people can inhale. Fibers can become embedded in lung tissue and cause asbestosis (a chronic breathing disorder that may be fatal), lung cancer, or mesothelioma (another form of cancer). For the most part, epidemiologists have documented these diseases only in workers exposed to high levels of asbestos over long times.
In 1907 health workers reported the first asbestos-related diseases, but it was not until around 1960 that the threat posed by asbestos was accepted as serious. In 1974 the Environmental Protection Agency (EPA) banned asbestos for most commercial use in the United States, and soon afterward launched a vigorous program to remove asbestos from commercial structures. However, American companies still ship many products containing asbestos to developing countries. Despite the ban and efforts to eliminate asbestos from our environment, it is still common in many buildings and as a component in urban dust.
Click on the arrow below to be taken to a video that shows some spectacular images of crystals that have different crystal habits. This video is one of many produced by the Envisioning Chemistry Project.