11.1: Front Matter
- Page ID
- 14564
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)What Is the Rock Cycle?
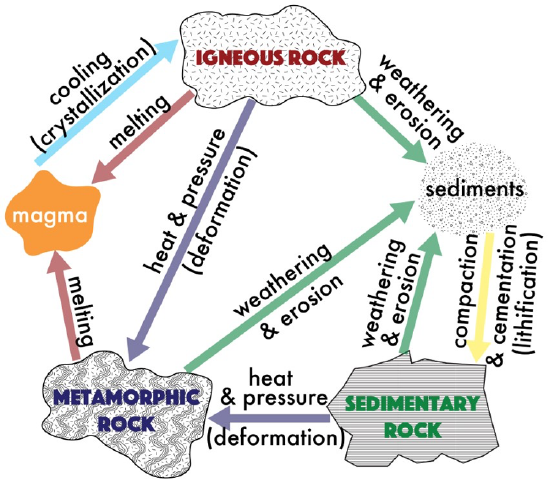
The rock cycle is a circular diagram that demonstrates the process by which rocks are continually recycled throughout geologic time. All rocks can be classified into one of three groups: igneous, sedimentary, or metamorphic. This rock classification is based on the origin of each of these rock types, or the processes that form the rock. The Rock Cycle (Figure 11.1), represents the processes that produce each of the rock types. In Chapter 7, we explored igneous rocks, which are produced through the melting of rock and eventual cooling of lava or magma; as cooling occurs, crystals may form. In this chapter, we will investigate sedimentary rocks, which are produced via the processes of weathering (the chemical and physical breakdown of material at Earth’s surface that produces sediments), erosion (the movement and deposition of sediments), and lithification (the compaction and cementation of sediments). In Chapter 13, we’ll look at metamorphic rocks, which are produced via the agents of change, primarily heat and pressure which typically result in the recrystallization of minerals.
What Are Sedimentary Rocks and Why Are They Important?
Sedimentary rocks are rocks that form at the Earth’s surface via the processes of weathering, erosion, and lithification. They are unique in that they record surface conditions and contain evidence of life, like fossils. Sedimentary rocks are the pages in which Earth’s history is written and can inform us about a wealth of subjects from the occurrence of ancient catastrophes to the productivity of life.
The identification of sedimentary rocks is more than applying names, since each name is a loaded term that conveys information regarding its history, where it was formed, potentially when it was formed, and the processes that led to its formation. Each sedimentary rock is a puzzle, and by identifying a set of rocks, how they are layered, the fossils contained within, and patterns in the rocks, a geologist can reconstruct an entire environment and ecosystem. Solving these puzzles can be useful to better understand the world around us. Additionally, important mined resources like fossil fuels, water, and salt are typically contained within sedimentary rocks.
Those who study sediments, sedimentary rocks, and their fossils may go by many titles: sedimentologist, sedimentary petrologist, or paleontologist. A sedimentary petrologist is a geoscientist who specializes in sedimentary rocks and their conditions of formation. Like many other geoscientists, working with other disciplines is common, with a heavy influence from both math and technology. Many are employed by universities where they teach and/or do research, and state and federal agencies, including geological surveys, like the California Geological Survey or United State Geological Survey (USGS). Additional career pathways are available in the private sector including in mining and natural resource extraction. Many of these career options require a college degree and postgraduate work. If you are interested, talk to your geology instructor for advice. We recommend completing as many math and science courses as possible (chemistry is incredibly important for mineralogy). Also, visit National Parks, CA State Parks, museums, gem & mineral shows, or join a local rock and mineral club. Typically, natural history museums will have wonderful displays of rocks, including those from your local region. Here in California, there are a number of large collections, including the San Diego Natural History Museum, Natural History Museum of Los Angeles County, Santa Barbara Museum of Natural History, and Kimball Natural History Museum. Many colleges and universities also have their own collections/museums.
Weathering
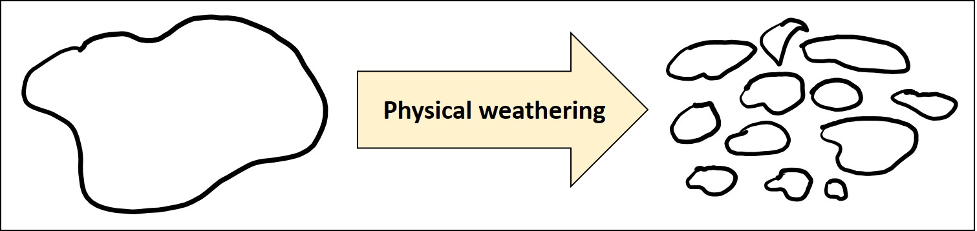
Sedimentary rocks are composed of the broken pieces of other rocks. The obvious place to start is examining how rocks are broken down, in a process called weathering. Weathering is the process from which sediments are derived or produced. There are two categories of weathering. The first, is physical or mechanical weathering, which results in rocks being physically broken into smaller pieces resulting in an overall size change (imagine hitting a rock with a hammer) (Figure 11.2). The second category is chemical weathering, which results in a fundamental chemical change of the original rock. The rock becomes altered at the atomic level (imagine dissolving salt in a glass of water). Oftentimes, both physical and chemical weathering will simultaneously affect a rock; however, the extent and rate of weathering is frequently dependent on the environment.
Physical or Mechanical Weathering
Abrasion, the most prevalent type of mechanical weathering, results from the collision, breaking, and grinding of rock during the movement of a fluid, either water or air. The size of the carried sediment depends on the fluid type and velocity. A fast fluid (e.g. a rapidly flowing river) can carry large particles and cause large amounts of abrasion. A slow fluid (e.g. a calm stream) would only cause a small amount of abrasion. The density of the fluid also controls the size of particles that can be transported. Denser fluid, like water, can carry larger particles, while less dense fluids, like air, carry smaller particles.
Another common method of mechanical weathering occurs when water seeps into a crack in the rock and freezes; this is called frost wedging or frost heaving. Water is unique in that it expands when frozen, which puts pressure on the rock and can split boulders (Figure 11.3).

The addition and subtraction of heat or pressure can also cause rock to break; this is called exfoliation. This breakage can also occur with rocks when they cool very quickly or immense pressure is released. Exfoliation of the granite in Yosemite National Park results in many of the rockfalls for which the park is famous (Figure 11.4).

Chemical Weathering
Rocks can be chemically weathered, usually by one of three common reactions. Water is frequently involved as it is good at inducing a chemical change. The first, which you are probably familiar with, is called dissolution. In this case, a mineral or rock is completely broken apart in water into chemical ions. These ions are then transported with the water and redeposited as the concentration of ions increases, normally because of evaporation (this is accomplished through a process called chemical precipitation, which is different than the rain type of precipitation). Table 11.1 illustrates the chemistry of calcite, which is prone to dissolution.
Chemical weathering can also change the mineralogy and weaken the original material, again through the agent of water. A mineral can undergo hydrolysis, which occurs when a hydrogen atom from a water molecule replaces the cation in a mineral. Typically, this alters minerals like feldspar into a softer clay mineral (like kaolinite).
Thirdly, a mineral can also undergo oxidation, which is when oxygen atoms alter the valence state of a cation. This normally occurs on a metal that is freshly exposed to atmospheric oxygen and is commonly known as rusting (Figure 11.5). Table 11.1 illustrates the chemistry of significant minerals and their chemical changes.

| Chemical Breakdown of Common Minerals | ||
|---|---|---|
| Chemical change | Process | Chemical reaction |
| Pyrite to hematite | Oxidation | 2FeS2 + 7O2 + 2H2O => 2Fe2+ + H2SO4 + 2H+ |
| Pyroxene to iron oxide | Oxidation | Fe2+SiO3 + O2 + H2O => FeO(OH) + SiO2 |
| Calcite to calcium & bicarbonate ions | Dissolution | CaCO3 + H+ + HCO3- => Ca2+ + 2HCO3- |
| Potassium feldspar to clay | Hydrolysis | 3KAlSi3O8 + 3H2O => Al2Si2O5(OH)4 + 4SiO2 +2K+ +2OH- |
| Olivine to serpentine | Hydrolysis + Oxidation = Serpentinization |
Fe2SiO4 + 4H2CO3 => 2Fe2+ + 4HCO3- + H4SiO4 2Fe2+ + ½O2 + 2H2O + 4HCO3- => Fe2O3 + 4H2CO3 |
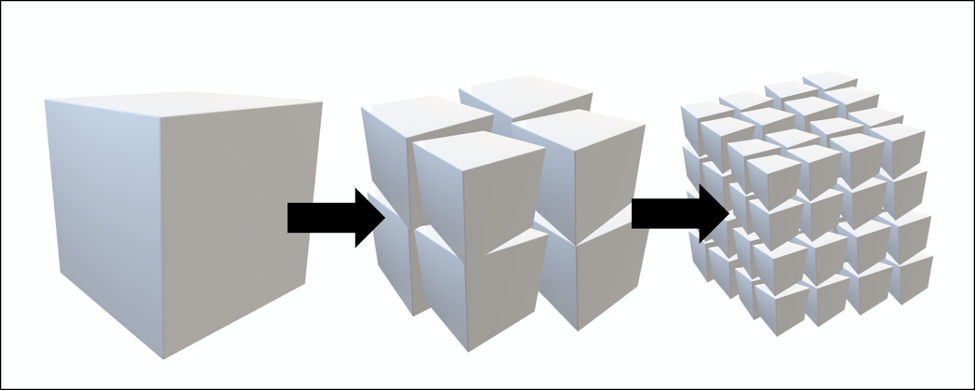
Chemical and mechanical weathering often work together to increase the overall rate of weathering. Chemical weathering weakens rocks, which makes them more prone to breaking physically, while mechanical weathering increases the surface area of the sediment, which increases the surface area that is exposed to chemical weathering (Figure 11.6).
Environments with multiple types of weathering typically result in rocks that break down quickly. Humid climates, with abundant water, will generally encourage changes faster than drier climates. In addition, we must also consider the original mineralogy of the rocks, as this can also play an important role in weathering potential (Table 11.2).
Finally, biology – plants, animals, and yes, even humans – can cause significant amounts of weathering, sometimes referred to as biological weathering. This can be done both physically and chemically. For example, trees put down roots through joints or cracks in the rock and, as the tree grows, the roots gradually pry the rock apart. Additionally, some animals bore into rocks for protection either by force or through the secretion of acid which dissolves the rock. Even bacteria, algae, and lichens produce chemicals that can break down rock.
| Relative Stability of Common Minerals Under Weathering | ||
|---|---|---|
| Mineral Name | Relative Stability | Rate of Weathering |
| Hematite | Most stable | Slowest |
| Quartz |  |
 |
| Clay minerals | ||
| Muscovite mica | ||
| Potassium feldspar | ||
| Biotite | ||
| Na-Plagioclase feldspar | ||
| Amphibole | ||
| Pyroxene | ||
| Ca-Plagioclase feldspar | ||
| Olivine | ||
| Calcite | ||
| Halite | Least stable | Fastest |
Erosion, Transportation, and Deposition
After sediments are produced via weathering, they then undergo erosion. Erosion includes both the movement of sediment, transportation via the agents of transportation (wind, water, ice, gravity), and the deposition of sediment in distinct areas referred to as depositional environments. Both sediment and the landscape are affected by the agents of transportation.
Sedimentary Depositional Environments
A depositional environment is the accumulation of chemical, biological, and physical properties and processes associated with the deposition of sediments that lead to a distinctive suite of sedimentary rocks. Sedimentary environments are interpreted by geologists based on clues within, such as rock types, sedimentary structures, trace fossils, and fossils. Geologists then compare these clues to modern environments to reconstruct ancient environments.
The numerous depositional environments found on earth can be broken into three major categories: 1) terrestrial or continental environments, common environments we find on land, 2) marine environments, those that are in the ocean, and 3) coastal or transitional environments, those at the interface between the first two (Figure 11.7).
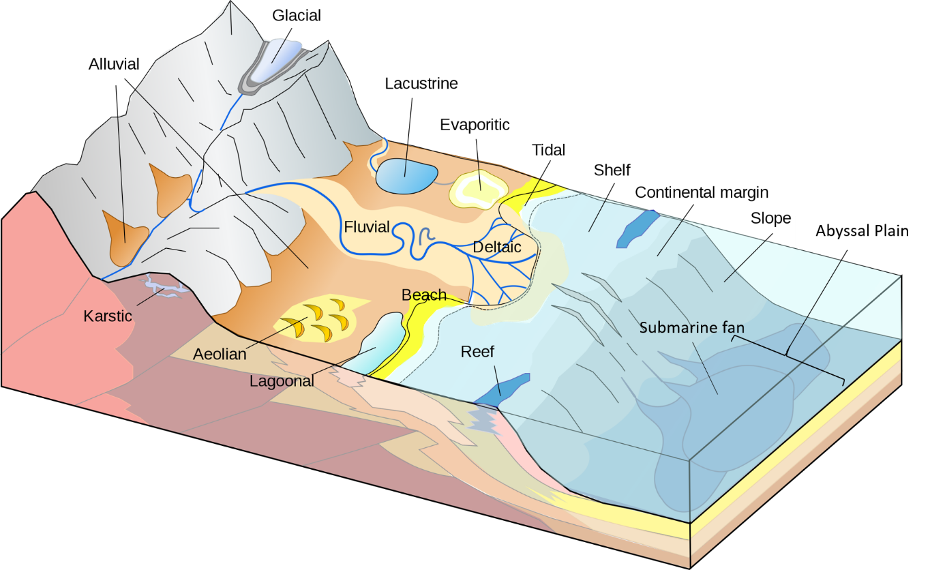
Terrestrial (Continental) Environments
There are many different environments on the continents, but consider how they are separated between those that are dominated by the deposition of sediments and those dominated by the erosion of sediments. Erosion occurs in high altitude areas and, although continents are overall topographically elevated compared to the oceans, there are many consistent areas on the continent with distinctive depositional properties. Continental depositional environments are dominated by clastic sedimentary rocks (see the Clastic Sedimentary Rock section), largely because of their proximity to the source of the sediments.
Glacial depositional environments are controlled mostly by the weathering and erosion by glaciers and glacial meltwater. Glaciers most commonly occur in areas that are both high elevation and/or high latitudes. Glaciers are fairly slow moving (centimeters a day) and normally travel short distances from their source, but they can cause immense mechanical weathering. Glaciers grind and bulldoze rock and create piles of poorly sorted sediment called moraines. Glaciers also produce a significant though fluctuating amount of meltwater, which flows through the moraines building a system of braided rivers. These rivers carry the small sediments further from the end of the glaciers into an area called the outwash plain, which consists of poorly sorted sediment.
Alluvial depositional environments are controlled primarily by gravity, although ephemeral river channels may develop during flash flooding. During these flood events extensive sediment will be moved from a high point, generally a mountain range, down to a lower elevation, producing alluvial fan deposits. These fan shaped deposits are similar to river deltas but occur on land. The sediment closest to the mountains will be larger in size, with smaller-sized sediment deposited towards the edges of the alluvial fan. Sediment here tends to retain a more angular shape, and little chemical weathering occurs.
Sediments that are deposited through the action of rivers are referred to as fluvial depositional environments. The gradient and discharge of a river can greatly control the shape of the river, how it flows, and how it deposits sediment. Rivers alter sediment both chemically and physically as well as sort the sediment since there is a limit to the size or particles a river can carry. Within a meandering river we see several different types of sediments, from the pebbles and gravel within the river channel to sandy point bars along the outer edge of the meander where the water slows. In addition, we see multiple types of sedimentary structures related to the flow of the river as well as those related to flood events.
We also have sediments deposited within lakes, which are called lacustrine depositional environments. Unlike rivers, lakes do not have rapidly flowing water; thus, there is significantly less movement of sediment and smaller particles. The sediment that accumulates in lakes can come from several sources, including rainwater carrying sediment into the lake from the shores, rivers that flow into the lake, and sediment that is transported by the wind. Once the sediment reaches the lake, it remains undisturbed so we see thin layers of fine sediment, with varying amounts of trace fossils.
Lastly, there are aeolian depositional environments, which are dominated by currents of wind rather than water. Since air is less dense than water, only smaller particles can be transported. In addition, wind is not restrained within distinctive channels like water and, therefore, the features of aeolian deposits are more widespread than those of fluvial deposits. Certain areas have predominant wind patterns, such that the wind is fairly consistent in direction and strength, which can generate significant sedimentary structures. Evaporitic environments are commonly associated in drier climates, where water is only present in the form of seasonal lakes (playa lakes) that undergo significant evaporation and sometimes leave behind chemical sedimentary rocks (evaporite deposits). See Table 11.3 for an overview of these environments.
| Characteristics of Terrestrial (Continental) Environments | |||
|---|---|---|---|
| Environment | Transport process(es) | Environments | Sediment types |
| Glacial | Gravity, moving ice, moving water | Valleys, plains, streams, lakes | Glacial till, gravel, sand, silt, clay |
| Alluvial | Gravity, intermittent moving water | Steep-sided valleys, talus | Coarse, angular fragments |
| Fluvial | Consistent moving water | Streams | Gravel, sand, silt, organic matter (swamps) |
| Aeolian | Wind | Deserts and coastal regions | Sand, silt |
| Lacustrine | Moving water (flowing into lake) | Lakes | Sand (near edges), silt, clay, organic matter |
| Evaporitic | Moving water (flowing into lake) | Lakes in arid regions (playa lake) | Salts, clays |
Transitional (Coastal) Environments
The interface between the continents and oceans are complicated areas that can be influenced by rivers, ocean currents, winds, waves, and tides. The sediments of coastal environments are a mixture of materials derived from the continents (clastic and organic) and those from the ocean (chemical and biochemical).
Shorelines that are influenced by strong daily tidal currents are called tidal mudflat depositional environments. Tides are currents that are the result of the gravitational forces exerted by the moon and the rotation of the earth. Shorelines that have strong tidal currents as well as seafloors with low gradients can have large areas that are submerged during high tide and exposed to air during low tide. These areas often have smaller particles than a normal shoreline since the tidal currents can pull fine-grained marine sediments into the area. In addition, the strong bidirectional currents, daily drying out, exposure to the elements, and abundant life create numerous sedimentary indicators of these environments.
Shorelines that are dominated by ocean currents are called beach depositional environments. Shorelines have constant winds blowing on and off shore that are the result of the difference in the way the land and water heat and cool through the day. These winds produce the waves that are iconic at the beach, but as these waves move onto shore they curve, mimicking the shape of the shore, and result in a longshore current, which runs parallel to the shore itself. This current carries and deposits sand along the beach. In addition, the wind can also produce dunes, which promote a diverse and complicated ecosystem.
| Characteristics of Coastal (Transitional) Environments | |||
|---|---|---|---|
| Environment | Transport process(es) | Environments | Sediment types |
| Tidal | Tidal currents | Tidal flats | Silt, clay |
| Beach | Waves, longshore currents | Beaches, spits, sand bars, barrier islands | Gravel, sand |
| Deltaic | Moving water | Deltas | Sand, silt, clay, organic matter (swampy areas only) |
Deltaic depositional environments are areas where rivers flow into the ocean, and produce deltas. As a river that is carrying material empties into the ocean, the water slows and deposits sediment. Most of the sediment is deposited at the mouth of the river with some spilling out into the surrounding areas building a distinctive fan of sediment. Since the sediment is coming from the river, the delta is largely a thick sequence of clastic sediment showing indications of the strong flow of the river. See Table 11.4 for more information regarding these environments.
Marine Environments
Marine depositional environments differ in multiple ways, but the resultant rocks are primarily controlled by the proximity and supply of continental sediment, the water depth, and the community of organisms that live in the area. The further an environment is from the shore, the less clastic sediment will be present, and the area will have a higher concentration of the chemical and biological sedimentary rocks that are formed within the ocean. In addition, some organisms in the right environmental conditions can produce huge amounts of skeletal material.
Shallow marine depositional environments are areas that are close to shore, but always submerged. These areas have a significant amount of mature clastic sediment along with marine algae (like seagrass) as well as skeletal material from animals like coral, echinoderms (sea urchins, sand dollars, sea stars), and mollusks (clams and snails). These areas can have a significant variation in their energy level, from high-energy shallow areas influenced by waves to low-energy deeper areas only influenced by large storms. A better understanding of the relative water depth can often be determined in sedimentary rocks based on their sedimentary structures, in addition to the community of organisms and types of trace fossils found in the rock, since these can be very sensitive to depth.
In warm tropical shallow water, we often find reef depositional environments. Reefs are formed through the growth of coral colonies building a large three-dimensional structure with their calcite skeletons. Corals can grow in many different marine environments, but they can only produce reefs when their symbiotic algae that live within their tentacles are able to photosynthesize effectively, resulting in more energy for the coral to grow faster. Reefs also create a barrier between shallow water environments and ocean currents, producing shallow, low-energy environments called lagoons. These lagoonal environments have thin layers of fine sediment that we would expect in quiet water along with chemical sedimentary deposits that are the result of evaporation.
Most of the ocean consists of deep marine depositional environments. Turbidite flows are fast-moving currents carrying a variety of sediment sizes down the continental slope to the abyssal plain (Figure 11.8). As the current slows, it can no longer carry the largest particles, so these will be deposited first. As the current continues to slow, progressively smaller particles are deposited on top of the bigger particles, forming submarine fans with graded bedding (Figure 11.9).

The remainder of the deep ocean contains areas beyond the reach of most clastic sediment other than the dust carried by the wind (red clay deposits). Therefore, sediment within the deep ocean is primarily produced chemically and biologically. The largest source of sediment in these deep settings is skeletal material from some of the smallest marine organisms (carbonate and siliceous oozes). Multiple types of single-celled organisms can produce shells composed of either silica or calcite. These shells are mostly produced in the surface waters that are bathed in sunlight permitting photosynthesis. When these organisms die, the shells then rain down into deeper water; this slow accumulation of sediment produces fine layers of biochemical sedimentary rocks. In some cases, these shells are dissolved or altered before they reach the bottom (which can be miles away) and are precipitated as chemical sedimentary rocks. Obviously, there is not a clear boundary between shallow and deep-water environments given the gradient of the ocean floor; however, deep marine depositional environments are normally thousands of feet deep and beyond the influence of even large storms. See Table 11.5 for more information regarding these environments.
| Characteristics of Marine Environments | |||
|---|---|---|---|
| Environment | Transport process(es) | Environments | Sediment types |
| Shallow marine water | Waves and tidal currents | Continental shelves and slopes, lagoons | Carbonates in tropical climates, sand/silt/clay elsewhere |
| Reefs | Waves and tidal currents | Reefs and adjacent basins | Carbonates |
| Lagoonal | Little transportation | Lagoon bottom | Carbonates in tropical climates |
| Submarine fan | Underwater gravity flows (turbidity currents) | Continental slopes, abyssal plains | Gravel, sand, silt, clay (turbidites) |
| Deep marine water | Ocean currents | Deep-ocean abyssal plains, trenches | Clay, carbonate mud (carbonate ooze), silica mud (siliceous ooze), red clays |
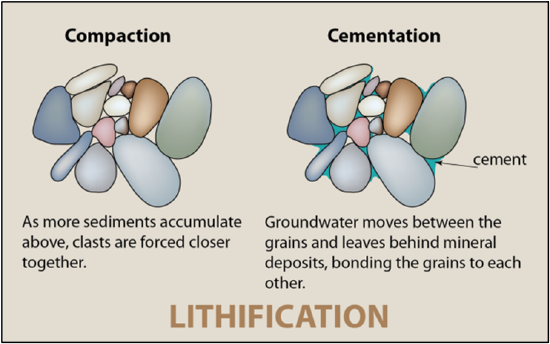
Lithification
The transformation of sediments into sedimentary rock happens during lithification. As sediments accumulate in the depositional environments, they are buried under more and more sediment. The sediments begin to compact under their own weight. Compaction, the squishing, of sediments, causes migration of water with dissolved ions. These ions become a natural glue, called cement, which precipitates and sticks sediments together, in a process called cementation. Types of cement include carbonate (calcite), iron-oxide (hematite), and silica (quartz). Compaction and cementation must both occur for a sedimentary rock to form; without lithification, the sediment will not transform into a true rock (Figure 11.10).
Sedimentary Structures
Sedimentary rocks often show distinctive patterns, called sedimentary structures, that reflect events or conditions during deposition. These patterns in the rocks can be very informative to geologists attempting to reconstruct the environment in which a sedimentary rock was formed. Many sedimentary structures are useful as “up-indicators”, meaning geologists use them to interpret where the surface of the Earth was in the geologic past. This is particularly useful if the layers have been deformed, bent or broken through tectonics.
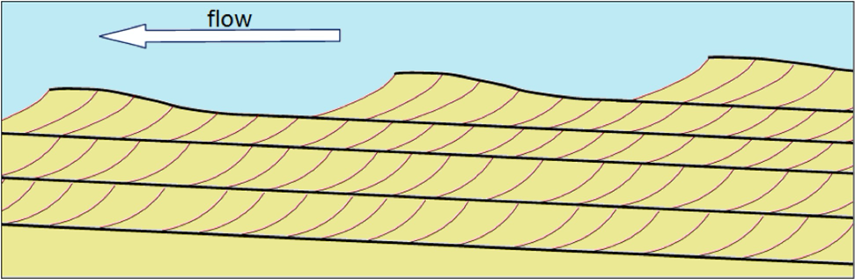
Most students are familiar with ripples and dunes (mega-ripples). We would typically see these structures at the beach or in deserts. Ripples and dunes form as a result of a wind or water current. Current or asymmetric ripples form when a dominant current, which moves in one direction, is present. The current pushes sediment into a pile: on the down-current side the sediment is shadowed and protected from the wind or water current; however, erosion on the up-current side results in a shallow slope and deposition on the steeper, down-current side. If you cut a ripple or dune in half and look at it in profile, you will see inclined layers of sediment building up on the steep down current side of the ripple (Figure 11.11).
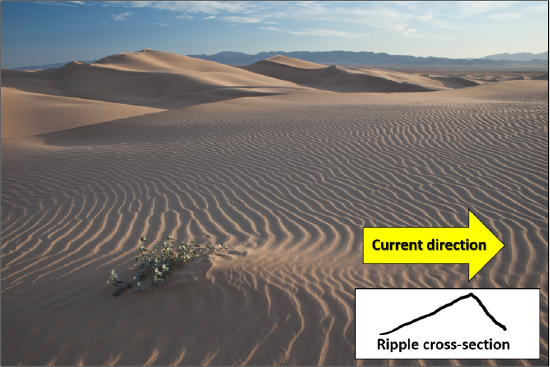
We often see multiple layers of beds consisting of these inclined layers, called cross-beds, which represent multiple generations of migrating ripples or dunes. Both ripples and cross-beds indicate the presence and direction of the current in an environment (Figure 11.12). Visit this site to see cross-beds on a sample from the Precambrian Johnnie Formation in southeastern Death Valley.
Wave-formed, symmetric, or oscillation ripples are commonly found in shallow waters, beaches, or tidal flats where water oscillates back and forth with wave-action. In cross-section, these ripples are symmetric and have long relatively straight crests (Figure 11.13).

Mud cracks, covered and preserved cracks that are the result of the drying of wet mud, and raindrop impressions, covered and preserved impacts of raindrops in soft mud, are both sedimentary structures that inform us about the sediment, water content, and exposure at the surface above water level.
Some sedimentary structures indicate changes in the strength of a current. Imagine a fast-moving current carrying a variety of sediment sizes; if the current slows, it will no longer be able to carry the largest particles and they will be deposited first. Then, as the current continues to slow, progressively smaller particles are deposited on top of the bigger particles, forming a sedimentary deposit called a graded bed (Figure 11.9). This graded bed is a sedimentary layer with larger clasts on the bottom and smaller clasts on the top. These types of beds can be the result of floods in a river, storms in the ocean, or turbidite flows.
Related to sedimentary structures are trace fossils, which are patterns in the rocks that are caused by the activity of organisms. Trace fossils indicate different aspects about the environment depending on the trace and the identity of the tracemaker. Traces can be terrestrial (e.g. footprints, burrows or dens, or the traces of roots) and can inform us about the climate, ecosystem, and the development of soils. Traces can also be found in freshwater and marine environments (e.g. burrows, borings, footprints, or feeding traces) and tell us about the sediment, chemistry of the pore water, and the life that lived within it. Visit this site to see trackways from the Pliocene of Death Valley National Park.
Clastic Sedimentary Rocks
How Do I Recognize a Clastic Sedimentary Rock?
Sedimentary rocks can be subdivided into two major groups based on the processes responsible for their formation: clastic (detrital) sedimentary rocks and non-clastic sedimentary rocks. Non-clastic (chemical, organic, biochemical) rocks are determined by their major chemical composition; however, if the sedimentary rock is composed of the broken pieces of other rocks, largely produced via physical weathering, the rocks are referred to as clastic or detrital sedimentary rocks. In a clastic sedimentary rock, the classification will primarily focus on the clast properties, including the clast size, overall shape (well-rounded to angular), and sorting of the clasts within the rock (uniform clast size to a range of sizes) (Figure 11.14).
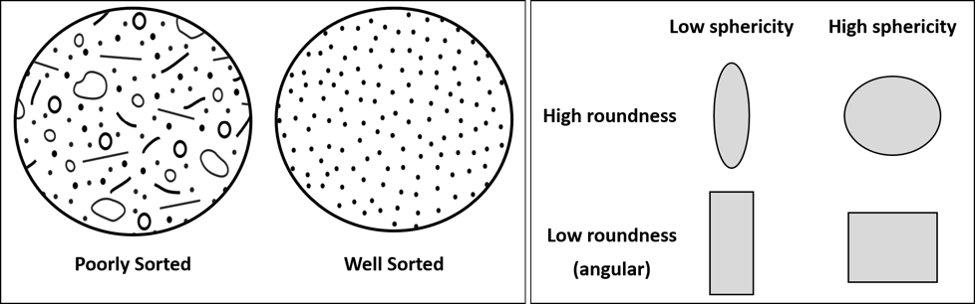
Recall that weathering and erosion generally occur in areas of high elevation, such as the mountains, while deposition occurs in lower areas such as valleys, lakes, or the ocean. Sediment is transported to the area of deposition via the agents of transportation: ice, water, air or gravity. During transportation, sediment changes, often significantly. Geologists (and you!) can recognize the amount of change and the distance the material has traveled, and the transport mechanism, by looking at the maturity of the sediment. Sediment maturity refers to the size, shape, and the general mineral composition of the sediment.
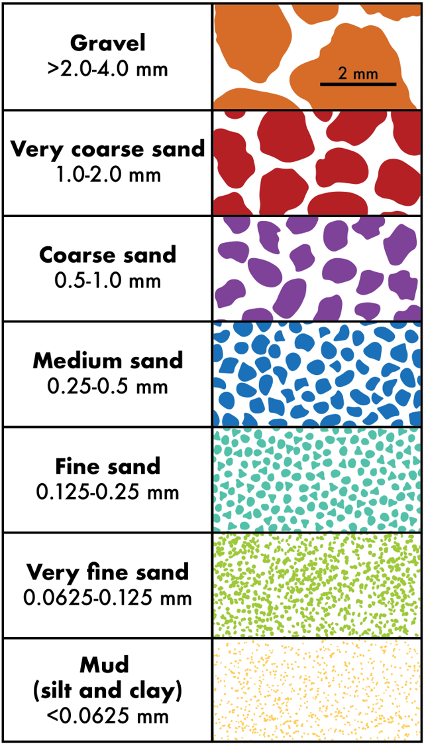
The size of sediments can be described using three broad categories: gravels, sands, and muds (Figure 11.15). Gravels and sands are visible, whereas muds are too small to see with the naked eye and typically need magnification to distinguish individual clasts.
The further sediments travel from their original source, the smaller, more rounded, sorted, and uniform they become (Figure 11.16). Consider your own life or educational journey: the more experiences you collect, the more well-rounded you become. For example, prior to graduation, most community colleges require you to complete numerous general education courses; this requirement is to facilitate your development, much like weathering and erosion, and to help you become a more well-rounded student and person. Secondly, much like each of us, our differences are in part a result of our experiences. The differences of clastic rocks are related to the various depositional environments, and these environments, and the distance traveled, can be interpreted based on the characteristics of the sediment.

Identifying the Different Clastic Sedimentary Rocks
Sedimentary breccia is an immature sedimentary rock with a poorly sorted mixture of clay, sand, and angular pebbles (gravel-sized) (Figure 11.17). The mineralogy of the clasts (sand and pebbles) often varies depending on the original source rock. Sedimentary breccias are typically found in continental environments, such as ancient landslides, or alluvial fans. The poorly-sorted, and angular nature of the gravel indicates the rock has only been transported a short distance, likely via gravity or inconsistent water.

Conglomerate is an immature sedimentary rock, with a poorly sorted mixture of clay, sand, and rounded pebbles (gravel-sized) (Figure 11.18). The mineralogy of the clasts (sand and pebbles) varies depending on the original source rock. Conglomerates are typically found in continental environments, such as ancient landslides, alluvial fans, or pebble beds in rivers. The poorly-sorted nature of the gravel indicates the rock has only been transported a short distance, but the rounded grains suggest some transportation, generally via a consistent water source.

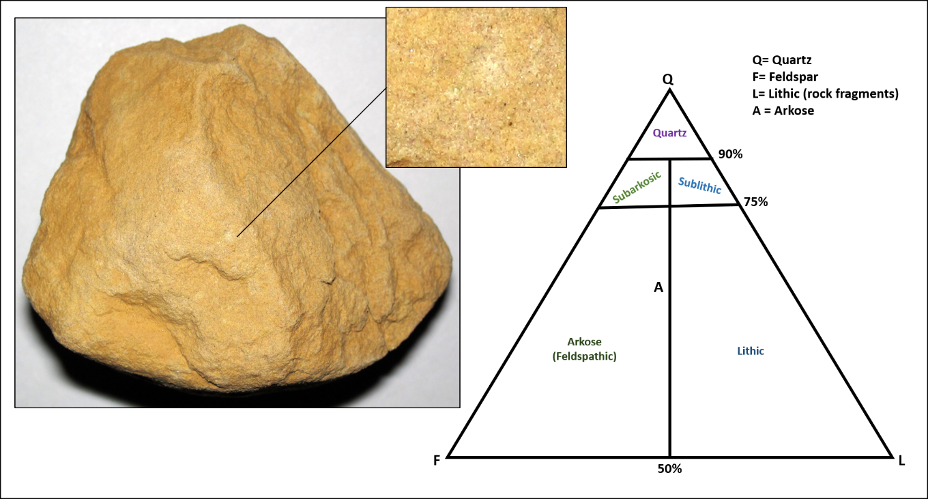
A sandstone consists of sand-sized clasts and is typically gritty to the touch (think about how sandpaper feels). These clasts vary in shape and mineralogy. Some sandstones may contain many minerals, while others may contain only quartz. Certain sandstone varieties, like arkose, are relatively immature and typically have more angular, moderately sorted, sand grains composed dominantly of the feldspar minerals (Figure 11.19). Arkose sandstone is typically associated with continental environments, like alluvial fans. Quartz sandstone is mature, as it contains well-rounded, well-sorted pure quartz grains. Quartz sandstone is typical of continental and transitional environments, like desert dunes or beaches. Lithic sandstones contain an abundance of lithic (rock) clasts. The ternary diagram in Figure 11.19, right, allows a sedimentary petrologist to carefully classify a sandstone based on its mineral composition.
Mudrocks, which are composed of varying amounts of silt or clay-sized particles, include a number of different named rocks. Silts and clays are very small and will likely need magnification to be visible, perhaps beyond what your hand lens can provide. In general, silts may feel slightly gritty; a good test for this in the field is to rub the rock gently on your front tooth. Your teeth are surprisingly sensitive, and will pick up the slight grit of a silt in a siltstone. If the rock is a mudstone or shale (contains layering, or fissility), the rock will contain dominantly clay-sized sediment and will feel smooth to the touch and on your tooth (Figure 11.20). Should you want to try this with your samples, check with the instructor first. Overall, mudrocks are extremely mature, and are made from the smallest particles, carried by wind or barely moving water. Silt and clay-sized sediment are typically thousands of miles from their original source rock.

Non-Clastic Sedimentary Rocks
How Do I Recognize a Non-Clastic Sedimentary Rock?
If the sedimentary rock is the product of chemical weathering, it will be referred to as non-clastic sedimentary rock, an all-inclusive term which covers sedimentary rocks produced via chemical, biochemical or organic processes. In a non-clastic rock, the classification is based primarily on the composition of the material and how it developed.
Chemical and Biochemical Sedimentary Rocks
In general, chemical sedimentary rocks typically have a crystalline appearance and form through the inorganic precipitation of minerals from a fluid. If the ions present within a fluid (water) become very concentrated, either by the addition of more ions or the removal of water (by freezing or evaporation), then crystals begin to form. In this case, identification of the type of sedimentary rocks is based on the minerals present.
In biochemical rocks, organisms typically facilitate the precipitation of these minerals from water. An example of biochemical precipitation is the formation of skeletal minerals in many organisms: from starfish and clams that grow calcite, to sponges that grow silica-based material, to humans that have bones and teeth made of hydroxyapatite. In many cases, it is hard to differentiate whether a mineral was formed organically or inorganically. Organic sedimentary rocks are those formed mostly from carbon-based organic material produced by ancient life.
Carbonate Sedimentary Rocks
Carbonates are one of the most important groups of sedimentary rocks. They can result in distinctive landscapes, called karst topography (more on this in Groundwater) and human hazards like sinkholes.
Limestone, one of the most abundant sedimentary rocks, is composed of the carbonate mineral calcite. If you remember our mineral unit, calcite undergoes dissolution in acids, and will bubble, fizz or effervesce, when dilute HCl is applied (Figure 11.21). There are many varieties of limestone and all vary greatly in appearance; however, all varieties will fizz or bubble when dilute HCl is applied. Limestone can form inorganically from a supersaturation of calcium and carbonate ions in water in a range of environments from caves to tropical beaches, although most limestone is marine in origin.

Crystalline limestone is typically inorganic in nature and consists of crystals of calcite or microcrystalline masses of calcite. It can be light gray to darker gray in color and the calcite crystals will sparkle when the sample is moved around under a light. Typically, crystalline limestone is indicative of a deep marine environment, but there are also lacustrine crystalline limestones. Micrite, a common limestone variety, is composed of limey mud (Figure 11.22).

Coquina often has a biologic component and is composed exclusively of shell or coral fragments (sometimes referred to as shell hash). Coquina looks a bit like dried oatmeal or a granola bar (Figure 11.23).

Fossiliferous limestone will contain fossils, but unlike coquina will not be entirely made of fossils. Oftentimes, between the fossils there will be calcite-rich mud (Figure 11.24). Typically, the fossils are marine in origin and include shells, crinoids, or other invertebrate marine life.

Oolitic limestone is composed of ooids (a fun geology word!), small calcite spheres that typically form in shallow, warm, highly agitated marine environments (Figure 11.25).

Chalk also has a biochemical origin and is made entirely of the small tests (shells) of marine organisms called coccolithophores, or coccoliths for short. These organisms are incredibly small, and can only be viewed under extreme magnification (Figure 11.26). Remember writing with chalk on a chalkboard? This rock will be powdery and leave a mess on your hands.

Travertine and tufa are both unique limestone varieties as they form in terrestrial (continental) environments. Travertine is commonly associated with hot spring environments, where supersaturated alkaline waters are geothermally heated (Figure 11.27).

The most famous hot spring travertine deposits are from Italy. Here in the US, the largest deposits are located in Mammoth Hot Springs in Yellowstone National Park. In California, Lassen Volcanic National Park has smaller deposits of travertine. Travertine can also be associated with caves and speleothems. Tufa commonly forms when carbonate minerals precipitate out of ambient temperature water, usually water that has not been geothermally heated. Here in California, there are beautiful tufa spires in Mono Lake and the Trona Pinnacles (Figure 11.28).

Dolostone is made from microscopic crystals of the mineral dolomite (Figure 11.29). Although also a carbonate mineral, dolomite only weakly reacts to dilute HCl. You must scratch and powder dolostone to increase the surface area to see the reaction with acid. Even after all that work, the reaction is muted (and underwhelming) when compared with calcite’s reaction. Current research suggests dolostone forms through secondary alteration of limestone and while we classify it as a chemical sedimentary rock it may have biochemical origins.

Siliceous Sedimentary Rocks
Chert is composed of microcrystalline varieties of quartz, and thus it has properties that are associated with quartz itself, such as conchoidal fracturing and hardness greater than glass (Figure 11.30). Typically, cherts are identified based on their appearance; for example, jasper is often red chert, flint is gray chert, and agate are banded cherts. Chert typically forms in deep marine environments from siliceous material that is either inorganic (silica clay) or biologic, a siliceous ooze formed from the skeletons of sponges and single-celled organisms like diatoms and radiolarians. Chert may also form petrified woods; these cherts form due to chemical changes during the heat and pressure of burial (diagenesis). Deep marine chert can be bedded or found as nodules in limestone layers. In prehistoric times, chert was often used for the construction of tools, like blades, where obsidian (volcanic glass) was unavailable.

Evaporite Sedimentary Rocks
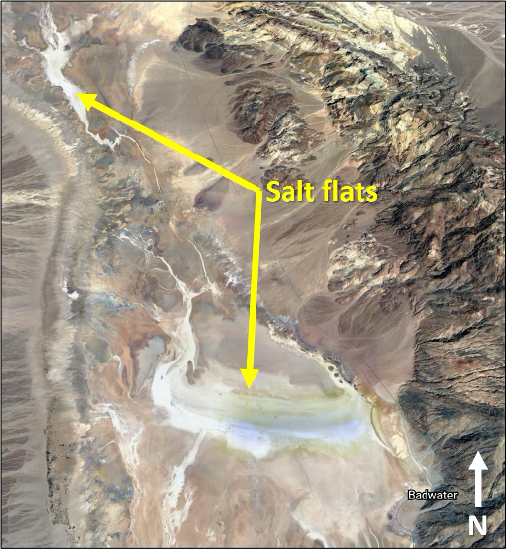
Evaporites are formed via the precipitation of minerals from the evaporation of water, in either restricted marine basins, playa lakes, or salt flats, like those of Death Valley, CA (Figure 11.31). Many of the evaporite deposits in California are geologically young. Rock salt, an aggregate of the mineral halite, may retain its cubic form and will vary in color. Rock gypsum (gypstone), an aggregate of the mineral gypsum, will typically be soft and may be massive or fibrous in appearance. Many of the evaporites contain significant economic minerals; for example, salt domes can be important in the search for petroleum.
Organic Sedimentary Rocks
Organic sedimentary rocks are rocks that consist mostly of organic carbon and are associated with significant biological activity. Other sedimentary rocks, such as limestone and shale (like oil shale) can contain carbon, but at much lower concentration. The most common organic sedimentary rock is coal. Coal is formed from the preservation and compaction of abundant plant material, buried in areas where oxygen is lacking (anoxic environments), such as swamps, wetlands, and bogs. All coal is combustible, and therefore can be ignited and burned; however, not all coal burns the same. In fact, there are grades of coal, which reflect increasing compaction or lithification.
Peat, typically dark brown (like potting soil), will contain obvious pieces of vegetation, and is typically the precursor to coal (Figure 11.32). In Ireland and Scotland there are peat bogs, from which the peat is mined, cut into bricks, dried, and used for the cooking and heating of homes.
Lignite coal is brittle with a low density (light heft) and has a matte brown or black color. It will also leave distinctive sooty marks on your fingers and hands. Typically, lignite coal contains 25%–35% carbon and has the lowest energy content of all coal ranks (Figure 11.32). Lignite coal deposits tend to be relatively young and were not subjected to extreme heat or pressure. Lignite accounts for about 10% of total U.S. coal production, most from North Dakota and Texas.
Bituminous coal will also be brittle with a low density (light heft), but its black color has a bit more shine to it. Typically, bituminous coal contains 45%–86% carbon (Figure 11.32). Here in the United States, bituminous coal is the most abundant of the coal varieties and accounts for almost 50% of our coal usage. Significant deposits are located in Wyoming, West Virginia, Pennsylvania, Kentucky, and Indiana. Much of the bituminous coal worldwide is from the Carboniferous period of the late Paleozoic (about 300 million years ago). Much of the bituminous coal mined here in the US is used to generate electricity and for processing iron to make steel.
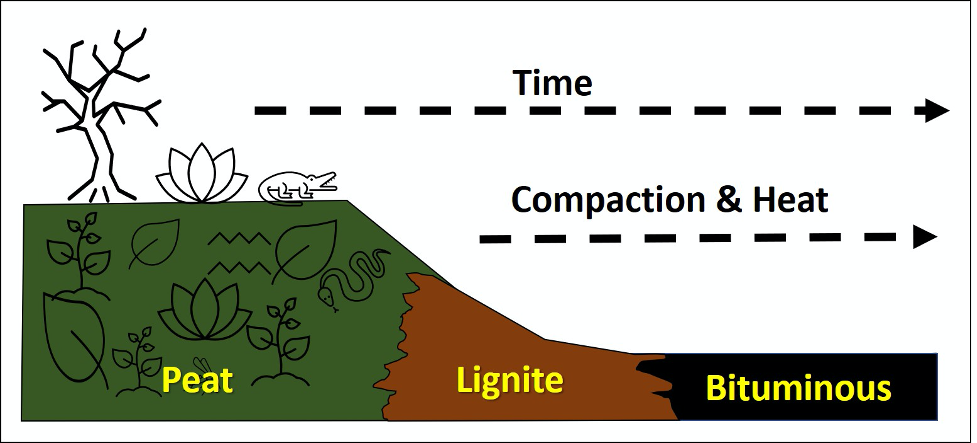
Anthracite coal is much harder than the previously outlined coal varieties. It is black in color with a submetallic luster. Anthracite accounts for <1% of coal mined in the US, with most mined from northeastern Pennsylvania (the “Anthracite Belt”). Anthracite typically contains 86%–97% carbon and is the highest grade of coal. Technically, anthracite has undergone so much heat and pressure that it is considered a metamorphic rock. We will discuss this rock further in the metamorphic rock chapter.
Attributions
- Figure 11.1: “Name That Sedimentary Rock” (CC-BY 4.0; Chloe Branciforte, own work)
- Figure 11.2: “Physical Weathering” (CC-BY 4.0; Chloe Branciforte, own work)
- Figure 11.3: Derivative of “Frost Wedging” (CC-BY 2.0; John Allen via Geograph and CC-BY 3.0; Julie Sandeen via Wikimedia Commons) by Chloe Branciforte
- Figure 11.4: “Half Dome Trek” (CC-BY 2.0; Ronnie Macdonald via Wikipedia)
- Figure 11.5: “Weathering” (CC-BY 3.0; Pollinator via Wikimedia Commons)
- Table 11.1: “Chemical Breakdown of Common Minerals” (CC-BY 4.0; Chloe Branciforte, own work)
- Figure 11.6: “Weathering and Surface Area” (CC-BY 4.0; Chloe Branciforte, own work)
- Table 11.2: “Relative Stability of Common Minerals Under Weathering” (CC-BY 4.0; Chloe Branciforte, own work)
- Figure 11.7: Derivative of “Main Depositional Environments” (CC-BY-SA 3.0; Mikenorton via Wikimedia Commons) by Chloe Branciforte
- Table 11.3: “Characteristics of Terrestrial Environments” (CC-BY 4.0; Chloe Branciforte, own work)
- Table 11.4: “Characteristics of Coastal Environments” (CC-BY 4.0; Chloe Branciforte, own work)
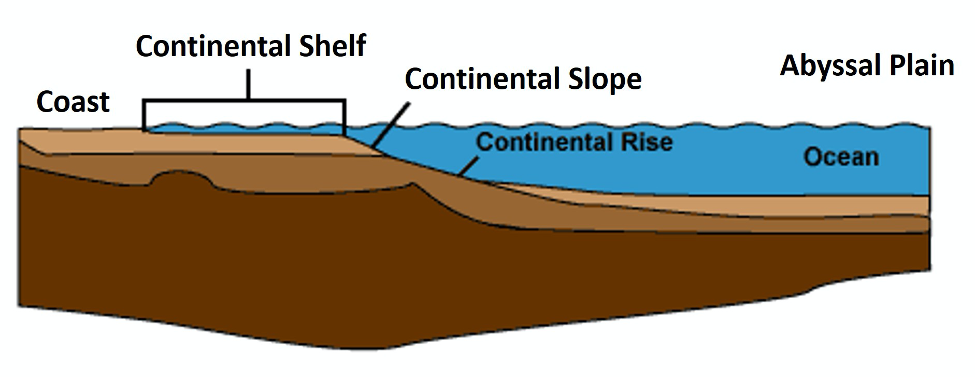
- Figure 11.8: Derivative of “Continental Shelf” (Public Domain; Interiot via Wikimedia Commons) by Chloe Branciforte
- Figure 11.9: Derivative of “Inverse Grading” (CC-BY-SA 3.0; Mikesclark via Wikipedia) and “Turbidite” (CC-BY 4.0; Chloe Branciforte, own work) by Chloe Branciforte
- Table 11.5: “Characteristics of Marine Environments” (CC-BY 4.0; Chloe Branciforte, own work)
- Figure 11.10: Derivative of “Lithification” (CC-BY 4.0; Karla Panchuk via OpenTextBC) by Emily Haddad.
- Figure 11.11: Derivative of “Cadiz Wilderness and Valley” (Public Domain; Bob Wick/BLM via Flickr) and “Current Ripple” (CC-BY 4.0; Chloe Branciforte, own work) by Chloe Branciforte
- Figure 11.12: “Cross-bedding” (CC-BY 4.0; Steven Earle via OpenTextBC)
- Figure 11.13: “Oscillation Ripple” (CC-BY 4.0; Chloe Branciforte, own work)
- Figure 11.14: Left, “Sediment Sorting” (CC-BY 4.0; Chloe Branciforte, own work); Right, “Sediment Sphericity and Roundness” (CC-BY 4.0; Chloe Branciforte, own work)
- Figure 11.15: “Sediment Grain Size” (CC-BY 4.0; Emily Haddad, own work)
- Figure 11.16: “The Journey of Sediment” (CC-BY 4.0; Chloe Branciforte, own work)
- Figure 11.17: “Chert Breccia” (CC-BY 2.0; James St. John via Flickr)
- Figure 11.18: “Quartz Pebble Conglomerate” (CC-BY 2.0; James St. John via Flickr)
- Figure 11.19: Derivative of “Hillsboro Sandstone” (CC-BY 2.0; James St. John via Flickr) and “Ternary Diagram” (CC-BY 4.0; Chloe Branciforte, own work) by Chloe Branciforte
- Figure 11.20: “Gray Shale” (CC-BY 2.0; James St. John via Flickr)
- Figure 11.21: “Effervescence” (CC-BY 4.0; Chloe Branciforte, own work)
- Figure 11.22: “Micrite Limestone” (CC-BY 2.0; James St. John via Flickr)
- Figure 11.23: “Coquina” (CC-BY 2.0; James St. John via Flickr)
- Figure 11.24: “Fossiliferous Limestone” (CC-BY 2.0; James St. John via Flickr)
- Figure 11.25: Derivative of “Oolitic Limestone” (CC-BY 2.0; James St. John via Flickr) by Chloe Branciforte
- Figure 11.26: Derivative of “Chalk” (CC-BY 2.0; James St. John via Flickr) and “Emiliania huxleyi Coccolithophore” (CC-BY 2.5; Alison R. Taylor/University of North Carolina Wilmington Microscopy Facility via Wikimedia Commons) by Chloe Branciforte
- Figure 11.27: “Travertine” (CC-BY 2.0; James St. John via Flickr)
- Figure 11.28: “Trona Pinnacles” (Public Domain; Bob Wick/BLM via Flickr)
- Figure 11.29: “Fossiliferous Dolostone” (CC-BY 2.0; James St. John via Flickr)
- Figure 11.30: Derivative of “Chert Nodule” (CC-BY 2.0; James St. John via Flickr) and “Flint” (CC-BY 2.0; James St. John via Flickr) and “Radiolarian Heliodiscus umbonatus” (CC-BY 2.0; Picturepest via Flickr) and “Marine Diatoms” (CC-BY-SA 3.0; Mogana Das Murtey and Patchamuthu Ramasamy via Wikimedia Commons) by Chloe Branciforte
- Figure 11.31: “Salt Flats of Badwater Basin” (CC-BY 4.0; Chloe Branciforte, own work via Google Earth)
- Figure 11.32: “Stages of Coal Formation” (CC-BY 4.0; Chloe Branciforte, own work)


