9.1: Environmental Toxicology
- Page ID
- 11809
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Environmental toxicology is the scientific study of the health effects associated with exposure to toxic chemicals and systems occurring in the natural, work, and living environments; the management of environmental toxins and toxicity; and the development of protections for humans, animals, and plants (Table below).
The table below lists top 20 substances, in order of priority, which are determined to pose the most significant potential threat to human health.This priority list is not a list of "most toxic" substances, but rather a prioritization of substances based on a combination of their frequency, toxicity, and potential for human exposure at various sites.
| 2013 RANK |
NAME |
|---|---|
| 1 | ARSENIC |
| 2 | LEAD |
| 3 | MERCURY |
| 4 | VINYL CHLORIDE |
| 5 | POLYCHLORINATED BIPHENYLS |
| 6 | BENZENE |
| 7 | CADMIUM |
| 8 | BENZO(A)PYRENE |
| 9 | POLYCYCLIC AROMATIC HYDROCARBONS |
| 10 | BENZO(B)FLUORANTHENE |
| 11 | CHLOROFORM |
| 12 | AROCLOR 1260 |
| 13 | DDT, P,P'- |
| 14 | AROCLOR 1254 |
| 15 | DIBENZO(A,H)ANTHRACENE |
| 16 | TRICHLOROETHYLENE |
| 17 | CHROMIUM, HEXAVALENT |
| 18 | DIELDRIN |
| 19 | PHOSPHORUS, WHITE |
| 20 | HEXACHLOROBUTADIENE |
Routes of Exposure to Chemicals
In order to cause health problems, chemicals must enter your body. There are three main “routes of exposure,” or ways a chemical can get into your body.
- Breathing (inhalation): Breathing in chemical gases, mists, or dusts that are in the air.
- Skin or eye contact: Getting chemicals on the skin, or in the eyes. They can damage the skin, or be absorbed through the skin into the bloodstream.
- Swallowing (ingestion): This can happen when chemicals have spilled or settled onto food, beverages, cigarettes, beards, or hands.
Once chemicals have entered your body, some can move into your bloodstream and reach internal “target” organs, such as the lungs, liver, kidneys, or nervous system.
What Forms do Chemicals Take?
Chemical substances can take a variety of forms. They can be in the form of solids, liquids, dusts, vapors, gases, fibers, mists and fumes. The form a substance is in has a lot to do with how it gets into your body and what harm it can cause. A chemical can also change forms. For example, liquid solvents can evaporate and give off vapors that you can inhale. Sometimes chemicals are in a form that can’t be seen or smelled, so they can’t be detected.
Detecting some forms of chemicals can be difficult. Solids and liquids are easier to recognize since they can be seen. Dusts and mists may or may not be visible, depending upon their size and concentration. Fumes, vapors, and gases are usually invisible.
What Health Effects Can Chemicals Cause?
An acute effect of a contaminant (The term “contaminant” means hazardous substances, pollutants, pollution, and chemicals) is one that occurs rapidly after exposure to a large amount of that substance. A chronic effect of a contaminant results from exposure to small amounts of a substance over a long period of time. In such a case, the effect may not be immediately obvious. Chronic effect are difficult to measure, as the effects may not be seen for years. Long-term exposure to cigarette smoking, low level radiation exposure, and moderate alcohol use are all thought to produce chronic effects.
For centuries, scientists have known that just about any substance is toxic in sufficient quantities. For example, small amounts of selenium are required by living organisms for proper functioning, but large amounts may cause cancer. The effect of a certain chemical on an individual depends on the dose (amount) of the chemical. This relationship is often illustrated by a dose-response curve which shows the relationship between dose and the response of the individual. Lethal doses in humans have been determined for many substances from information gathered from records of homicides and accidental poisonings.
Much of the dose-response information also comes from animal testing. Mice, rats, monkeys, hamsters, pigeons, and guinea pigs are commonly used for dose-response testing. A population of laboratory animals is exposed to measured doses under controlled conditions and the effects noted and analyzed. Animal testing poses numerous problems, however. For instance, the tests may be painful to animals, and unrelated species can react differently to the same toxin. In addition, the many differences between test animals and humans makes extrapolating test results to humans very difficult. A dose that is lethal to 50 percent of a population of test animals is called the lethal dose-50 percent or LD-50. Determination of the LD-50 is required for new synthetic chemicals in order to give a measure of their toxicity. A dose that causes 50 percent of a population to exhibit any significant response (e.g., hair loss, stunted development) is referred to as the effective dose-50 percent or ED-50. Some toxins have a threshold amount below which there is no apparent effect on the exposed population.
Some scientists believe that all toxins should be kept at a zero-level threshold because their effects at low levels are not well known. That is because of the synergy effect in which one substance exacerbates the effects of another. For example, if cigarette smoking increases lung cancer rates 20 times and occupational asbestos exposure also increases lung cancer rates 20 times, then smoking and working in an asbestos plant may increase lung cancer rates up to 400 times.
Environmental Contaminants
The contamination of the air, water, or soil with potentially harmful substances can affect any person or community. Contaminants (Table below) are often chemicals found in the environment in amounts higher than what would be there naturally. We can be exposed to these contaminants from a variety of residential, commercial, and industrial sources. Sometimes harmful environmental contaminants occur biologically, such as mold or a toxic algae bloom.
| Contaminant | Definition |
|---|---|
| Carcinogen | An agent which may produce cancer (uncontrolled cell growth), either by itself or in conjunction with another substance. Examples include formaldehyde, asbestos, radon, vinyl chloride, and tobacco. |
| Suspect Carcinogen | An agent which is suspected of being a carcinogen based on chemical structure, animal research studies, or mutagenicity studies. |
|
Confirmed Animal Carcinogen with Unknown Relevance to Humans |
An agent that is carcinogenic in experimental animals at a relatively high dose, by routes of administration, at sites, or histologic types, or by mechanisms that may not be relevant to worker exposure. Available epidemiologic studies do not confirm an increased risk of cancer in exposed humans. Available evidence does not suggest that the agent is likely to cause cancer in humans except under uncommon or unlikely routes or levels of exposure. |
| Teratogen |
A substance which can cause physical defects in a developing embryo. Examples include alcohol and cigarette smoke. |
| Mutagen | A material that induces genetic changes (mutations) in the DNA. Examples include radioactive substances, x-rays and ultraviolet radiation. |
| Neurotoxicant | A substance that can cause an adverse effect on the chemistry, structure or function of the nervous system. Examples include lead and mercury. |
|
Endocrine disruptor |
A chemical that may interfere with the body’s endocrine system and produce adverse developmental, reproductive, neurological, and immune effects in both humans and wildlife. A wide range of substances, both natural and man-made, are thought to cause endocrine disruption, including pharmaceuticals, dioxin and dioxin-like compounds, arsenic, polychlorinated biphenyls (PCBs), DDT and other pesticides, and plasticizers such as bisphenol A (BPA). Endocrine disruptors may be found in many everyday products– including plastic bottles, metal food cans, detergents, flame retardants, food, toys, cosmetics, and pesticides. Research shows that endocrine disruptors may pose the greatest risk during prenatal and early postnatal development when organ and neural systems are forming. |
The following are some environmental contaminants that can affect a community or an individual's health.
Arsenic is a naturally occurring element that is normally present throughout our environment in water, soil, dust, air, and food. Levels of arsenic can vary from place to place due to farming and industrial activity as well as natural geological processes. The arsenic from farming and smelting tends to bind strongly to soil and is expected to remain near the surface of the land for hundreds of years as a long-term source of exposure. Wood that has been treated with chromated copper arsenate (CCA) is commonly found in decks and railing in existing homes and outdoor structures such as playground equipment. Some underground aquifers are located in rock or soil that has naturally high arsenic content.
Most arsenic gets into the body through ingestion of food or water. Arsenic in drinking water is a problem in many countries around the world, including Bangladesh, Chile, China, Vietnam, Taiwan, India, and the United States. Arsenic may also be found in foods, including rice and some fish, where it is present due to uptake from soil and water. It can also enter the body by breathing dust containing arsenic, or through the skin, though this is not a major route of exposure. Researchers are finding that arsenic, even at low levels, can interfere with the body’s endocrine system. In several cell culture and animal models, arsenic has been found to act as an endocrine disruptor, which may underlie many of its health effects. Arsenic is also a known human carcinogen associated with skin, lung, bladder, kidney, and liver cancer.
Polychlorinated biphenyls, commonly called PCBs, are mixtures of up to 209 chlorinated compounds that do not occur naturally. They have no taste or smell. PCBs are persistent organic pollutants (POPs) and endocrine disruptors. The manufacture of PCBs was stopped in the U.S. in 1977 because of evidence they build up in the environment and can cause harmful health effects. But, before 1977, PCBs were used as insulation, as plasticizers, and in surface coatings, sealants, fire retardants, glues, inks, pesticides, and carbonless copy paper. PCBs don't break down easily in the environment and may remain there for very long periods of time. Studies indicate that PCBs are associated with certain kinds of cancer in humans. Women who were exposed to relatively high levels of PCBs in the workplace or ate large amounts of fish contaminated with PCBs had babies that weighed slightly less than babies from women who did not have these exposures.
Mercury is a naturally occurring metal, a useful chemical in some products, and a potential health risk. Mercury exists in several forms – the types people are usually exposed to are methylmercury and elemental mercury. Elemental mercury at room temperature is a shiny, silver-white liquid, which can produce a harmful odorless vapor. Methylmercury, an organic compound, can build up in the bodies of long-living, predatory fish. To keep mercury out of the fish we eat and the air we breathe, it's important to take mercury-containing products to a hazardous waste facility for disposal. Common products sold today that contain small amounts of mercury include fluorescent lights and button-cell batteries.
Although fish and shellfish have many nutritional benefits, consuming large quantities of fish increases a person’s exposure to mercury. Pregnant women who eat fish high in mercury on a regular basis run the risk of permanently damaging their developing fetuses. Children born to these mothers may exhibit motor difficulties, sensory problems and cognitive deficits. The poster below (published by the Maine Center for Disease Control & Prevention) identifies the typical (average) amounts of mercury in commonly consumed commercial and sport-caught fish.
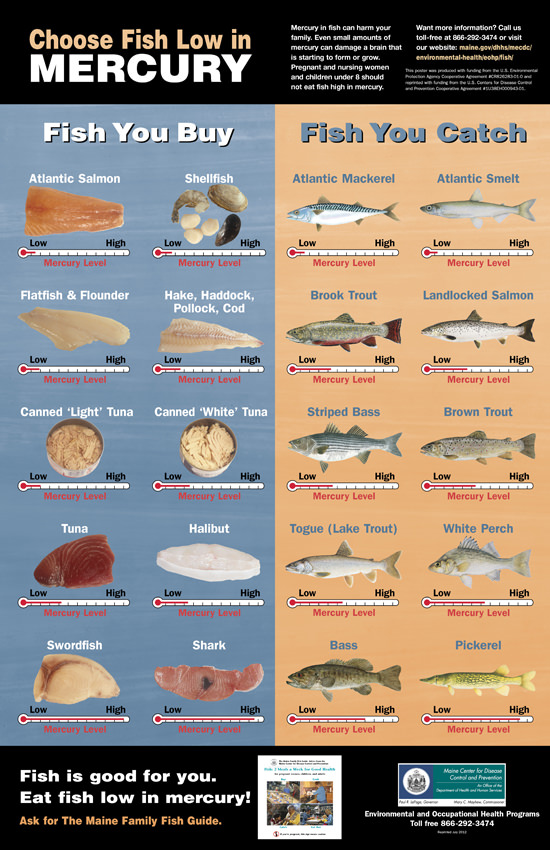
Figure \(\PageIndex{1}\): Mercury in Fish Poster
Bisphenol A (BPA) is a chemical produced in large quantities for use primarily in the production of polycarbonate plastics and epoxy resins. Polycarbonate plastics have many applications including use in some food and drink packaging, e.g., water and infant bottles, compact discs, impact-resistant safety equipment, and medical devices. Epoxy resins are used as lacquers to coat metal products such as food cans, bottle tops, and water supply pipes. Some dental sealants and composites may also contribute to BPA exposure. The primary source of exposure to BPA for most people is through the diet. Bisphenol A can leach into food from the protective internal epoxy resin coatings of canned foods and from consumer products such as polycarbonate tableware, food storage containers, water bottles, and baby bottles. The degree to which BPA leaches from polycarbonate bottles into liquid may depend more on the temperature of the liquid or bottle, than the age of the container. BPA can also be found in breast milk.
What can I do to prevent exposure to BPA?
Some animal studies suggest that infants and children may be the most vulnerable to the effects of BPA. Parents and caregivers, can make the personal choice to reduce exposures of their infants and children to BPA:
- Don’t microwave polycarbonate plastic food containers. Polycarbonate is strong and durable, but over time it may break down from over use at high temperatures.
- Plastic containers have recycle codes on the bottom. Some, but not all, plastics that are marked with recycle codes 3 or 7 may be made with BPA.
- Reduce your use of canned foods.
- When possible, opt for glass, porcelain or stainless steel containers, particularly for hot food or liquids.
- Use baby bottles that are BPA free.
Dioxins are a class of chemical contaminants that are formed during combustion processes such as waste incineration, forest fires, and backyard trash burning, as well as during some industrial processes such as paper pulp bleaching and herbicide manufacturing. The highest environmental concentrations of dioxin are usually found in soil and sediment, with much lower levels found in air and water. We are primarily exposed to dioxins by eating food contaminated by these chemicals. Studies have also shown that chemical workers who are exposed to high levels of dioxins have an increased risk of cancer. Other studies of show that dioxins can cause reproductive and developmental problems, and an increased risk of heart disease and diabetes.
Phthalates are a group of chemicals used to soften and increase the flexibility of plastic and vinyl. Polyvinyl chloride is made softer and more flexible by the addition of phthalates. Phthalates are used in hundreds of consumer products. Phthalates are used in cosmetics and personal care products, including perfume, hair spray, soap, shampoo, nail polish, and skin moisturizers. They are used in consumer products such as flexible plastic and vinyl toys, shower curtains, wallpaper, vinyl miniblinds, food packaging, and plastic wrap. Exposure to low levels of phthalates may come from eating food packaged in plastic that contains phthalates or breathing dust in rooms with vinyl miniblinds, wallpaper, or recently installed flooring that contain phthalates. We can be exposed to phthalates by drinking water that contains phthalates. Phthalates are suspected to be endocrine disruptors.
Lead is a metal that occurs naturally in the rocks and soil of the earth's crust. It is also produced from burning fossil fuels such as coal, oil, gasoline, and natural gas; mining; and manufacturing. Lead has no distinctive taste or smell. The chemical symbol for elemental lead is Pb. Lead is used to produce batteries, pipes, roofing, scientific electronic equipment, military tracking systems, medical devices, and products to shield X-rays and nuclear radiation. It is used in ceramic glazes and crystal glassware. Because of health concerns, lead and lead compounds were banned from house paint in 1978; from solder used on water pipes in 1986; from gasoline in 1995; from solder used on food cans in 1996; and from tin-coated foil on wine bottles in 1996. The U.S. Food and Drug Administration has set a limit on the amount of lead that can be used in ceramics.
Lead and lead compounds are listed as "reasonably anticipated to be a human carcinogen". It can affect almost every organ and system in your body. It can be equally harmful if breathed or swallowed. The part of the body most sensitive to lead exposure is the central nervous system, especially in children, who are more vulnerable to lead poisoning than adults. A child who swallows large amounts of lead can develop brain damage that can cause convulsions and death; the child can also develop blood anemia, kidney damage, colic, and muscle weakness. Repeated low levels of exposure to lead can alter a child's normal mental and physical growth and result in learning or behavioral problems. Exposure to high levels of lead for pregnant women can cause miscarriage, premature births, and smaller babies. Repeated or chronic exposure can cause lead to accumulate in your body, leading to lead poisoning.
Polyvinyl chloride (PVC) is an odorless and solid plastic. It is most commonly white but can also be colorless or amber. It can also come in the form of white powder or pellets. PVC is made from vinyl chloride. PVC is made softer and more flexible by the addition of phthalates. Bisphenol A (BPA) is also used to make PVC plastics. PVC contains high levels of chlorine. PVC is used to make pipes, pipe fittings, pipe conduits, vinyl flooring, and vinyl siding. When softened with phthalates, PVC is used to make some medical devices (including intravenous (IV) bags, blood bags, blood and respiratory tubing) and consumer products (raincoats, toys, shower curtains, furniture, carpet backing, plastic bags and credit cards). Most vinyl chloride produced in the United States is used to make PVC.
Exposure to PVC often includes exposure to phthalates, which are used to soften PVC and may have adverse health effects. Because of PVC’s heavy chlorine content, dioxins are released during the manufacturing, burning, or landfilling of PVC. Exposure to dioxins can cause reproductive, developmental, and other health problems, and at least one dioxin is classified as a carcinogen. Dioxins, phthalates, and BPA are suspected to be endocrine disruptors, which are chemicals that may interfere with the production or activity of hormones in the human endocrine system.
Formaldehyde is a colorless, flammable gas or liquid that has a pungent, suffocating odor. It is a volatile organic compound, which is an organic compound that easily becomes a vapor or gas. It is also naturally produced in small, harmless amounts in the human body. The primary way we can be exposed to formaldehyde is by breathing air containing it. Releases of formaldehyde into the air occur from industries using or manufacturing formaldehyde, wood products (such as particle-board, plywood, and furniture), automobile exhaust, cigarette smoke, paints and varnishes, and carpets and permanent press fabrics. Nail polish, and commercially applied floor finish emit formaldehyde.

Figure \(\PageIndex{2}\): Nail products are known to contain toxic chemicals, such as dibutyl phthalate (DBP), toluene, and formaldehyde.
In general, indoor environments consistently have higher concentrations than outdoor environments, because many building materials, consumer products, and fabrics emit formaldehyde. Levels of formaldehyde measured in indoor air range from 0.02–4 parts per million (ppm). Formaldehyde levels in outdoor air range from 0.001 to 0.02 ppm in urban areas.
Radiation
Radiation is energy given off by atoms and is all around us. We are exposed to radiation every day from natural sources like soil, rocks, and the sun. We are also exposed to radiation from man-made sources like medical X-rays and smoke detectors. We're even exposed to low levels of radiation on cross-country flights, from watching television, and even from some construction materials. You cannot see, smell or taste radiation. Some types of radioactive materials are more dangerous than others. So it's important to carefully manage radiation and radioactive substances to protect health and the environment.
Radon is a colorless, odorless radioactive gas. It comes from the natural decay of uranium or thorium found in nearly all soils. It typically moves up through the ground and into the home through cracks in floors, walls and foundations. It can also be released from building materials or from well water. Radon breaks down quickly, giving off radioactive particles. Long-term exposure to these particles can lead to lung cancer. Radon is the leading cause of lung cancer among nonsmokers, according to the U.S. Environmental Protection Agency, and the second leading cause behind smoking.

