7.11: Quiz Questions - Chapter 7 - Properties of Seawater
- Page ID
- 10261
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)1. It is currently estimated that 97.2% of water resources on Earth is in the world ocean. Most of the remainder is:
a. frozen in glaciers and ice caps.
b. trapped as groundwater and soil moisture.
c. stored in lakes, streams and reservoirs.
d. trapped as water vapor in the atmosphere.
2. Seawater is composed of water, dissolved matter (including solids and gas [as ions], and suspended matter (dust and organic residues). Of the ions in seawater (besides hydrogen and oxygen), the two most abundant elements are:
a. sodium and calcium
b. chlorine and sodium
c. calcium and sulphate (SO4)
d. magnesium and potassium
3. Water is a polar substance. Each molecule of water has a negative charge associated with its oxygen, and a positive charge with it hydrogen. This polar character is responsible for its properties to have high surface tension because water molecules stick together. This property is called:
a. cohesion.
b. adhesion.
c. capillary action.
d. a solution.
4. Water is a powerful solvent: A solvent is a substance that dissolves a solute (a chemically different liquid, solid or gas), resulting in a solution. Not everything dissolves equally in water. However, substances that are most easily dissolved are held together by:
a. covalent bonds.
b. metallic bonds.
c. ionic bonds.
d. Van der Waals forces.
5. pH is a measure of acidity and alkalinity of a water solution. Because of the natural buffering system of dissolved bicarbonate (+HCO3) dissolve in seawater:
a. seawater is slightly acidic.
b. seawater has a neutral water pH of 7.0.
c. seawater is generally always within a range of pH of 7.5 to 8.5.
d. seawater is highly salty with a pH of about 9.0 or higher.
6. How much energy would it require to heat 20 grams of ice at -20° C to steam at 120° C? Do the math!
| (Key info) Specific heat: ice = 0.5 cal/gm water = 1.0 cal/gm steam = 0.5 cal/gm Latent heat: ice to water: 80 cal/gm water to steam: 540 cal/gm |
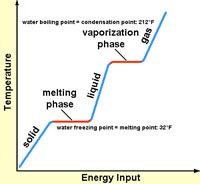 |
a. 3,400 calories
b. 7,350 calories
c. 12,980 calories
d. 29,600 calories
7. Lots of energy is released when:
a. water is converted to steam.
b. water evaporates on your skin.
c. ice melts to form water.
d. water vapor condenses in the air to form rain droplets.
8. As water evaporates into in air it cools the air and increases its humidity. As humid air rises it expands and cools and if it reaches the saturation point clouds form as water is forced to condense. The condensation of water releases a lot of energy, heating the air and causing clouds to rise into larger thunderstorms clouds. The water released falls as precipitation. How much water can the air hold to at at surface conditions before before it becomes saturated and can not absorb more water?
a. 2%
b. 4%
c. 8%
d. 12%
9. The salinity of seawater varies slightly from one part of the ocean to another, however, the average salinity of the oceans is about:
a. 4% (pph).
b. 17‰ (ppt).
c. 35‰ (ppt).
d. 40‰ (ppt).
10. Which of the relationships of temperature, salinity, and density of seawater is NOT true?
a. As temperature increases, density decreases.
b. As salinity increases, density increases.
c. As temperature changes, salinity remains the same.
d. As density increases, temperature increases.
11. Global data measurements of precipitation and evaporation show patterns of all EXCEPT which of the following.
a. The temperate regions receive more precipitation than tropical regions.
b. The polar regions have receive more precipitation than evaporates.
c. The temperate regions receive less precipitation than evaporates.
d. The tropics (equatorial region) receives much more rain than evaporates.
12. A thermocline is a layer of water at the ocean surface that prevents the upwelling and mixing of cool nutrient-rich water to ocean surface waters, reducing the production of primary plankton (food and nutrients for marine life). What is the best description of thermoclines based on latitude?
a. Polar regions have well-developed thermoclines in winter months.
b. Temperate regions have strong thermoclines in the winter months.
c. Tropical region have no thermocline in winter months.
d. Temperate regions have weak thermoclines (moderate in summer, less in winter).
13. Comparing oxygen carbon dioxide concentrations in the air compared with seawater, what of the following choices are true?
a. Seawater has higher concentrations of oxygen than the air.
b. Carbon dioxide (CO2) is much more soluble in water than oxygen, but concentrations of CO2 in the atmosphere are comparatively very low.
c. Wind and wave action reduces the exchange of oxygen and carbon dioxide (CO2) with seawater.
d. All of the above are true.
14. Sulfur dioxide (SO2) smells like rotten eggs. SO2 is released in large quantities by volcanic eruptions and by burning coal and petroleum. SO2 is extremely soluble in water where it combines with water molecules to form sulfate ions (-HSO4). When concentrated by evaporation in seawater, what happens?
a. It becomes sulfuric acid (H2SO4).
b. It precipitates as gypsum (CaSO4-2H2O) and anhydrite (CaSO4).
c. It is released back into the air as concentrated SO2.
d. All of the above.
15. Methane (CH4) has very low solubility in seawater, however, it is very abundant in sediments rich in organic mater. In cold settings, methane, carbon dioxide, and water form an unusual form of "flammable" ice called:
a. a clathrate.
b. sea ice.
c. permafrost.
d. all of the above.


