7.2: Specific Heat and Latent Heat Capacity of Water
- Page ID
- 10251
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Specific Heat and Latent Heat Capacity of Water
Specific Heat
Materials vary in their capacity to store thermal energy. For example, a material like copper will heat up much faster than water or wood. Specific Heat is a measure of the energy required to heat 1 gram of substance 1° C. Specific heat is recorded in "calories" for “mass in grams” (and “Joules for kg”).
Figure 7.11 compares the specific heat of various metals to the specific heat of ice, water, and steam. It takes significantly more energy to warm water than other materials, including both ice and steam. Because of water's high specific heat capacity, the oceans are capable of storing vast quantities of energy from solar heating. Heat absorbed in equatorial regions can be carried long distances and carried by ocean currents before being released in polar regions.
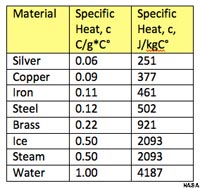
Figure 7.11. Comparison of the specific heat of various substances with ice, steam, and liquid water.
High Latent Heat Capacity of Water
When any material is heated to the temperature where it changes state (converting from solid to liquid, or liquid to gas), the temperature will remain the same until all the material changes state. Because it takes more energy to convert a substance from one physical state to another (solid to liquid, or liquid to gas), those transitions require a larger amount of energy. Latent heat is the heat required (measured in calories burned) to convert a solid into a liquid or vapor, or a liquid into a vapor, without a change of temperature. For instance a pot filled with water on the stove will gradually warm up until the water temperature approaches 212° F (or 100° C)—it will stay at that temperature until all the water has boiled away. The same is true as water freezes. As water cools it will reach 32°F (or 0° C) is will stay at that temperature until all the water freezes (Figure 7.12).
| To convert 1 gram of ice at 0° C to 1 gram of water at 0° C requires 80 calories. |
| To convert 1 gram of water at 100° C to 1 gram of steam at 100° C requires 540 calories. |
When any material is heated to the temperature where it changes state, the temperature will remain the same until all the material changes state. That means ice water will remain at 0° C (32° F) until all the ice is melted. The same thing applies when cooling the materials. The reason is that energy must be expended to change the state from solid to liquid or from liquid to gas. Likewise, energy must be withdrawn to change the state when cooling the material. The amount of energy required is call the latent heat of freezing or boiling.
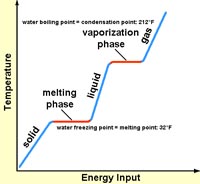
Figure 7.12. Diagram showing heat required to heat water from solid ice to liquid water and to vapor (steam). It take both specific heat and latent heat, released in stages, to convert ice to steam!
Specific Heat and Latent Heat Capacity of Ice, Water, and Steam Illustrated: (using charts listed above)
Example: How much energy would it require to heat 100 grams of ice at -10° C to steam at 120° C?
| Calculation process: | Energy required: |
| To raise 100 grams of ice at -10° C to 0° C requires: (0.5 cal/g [specific heat of ice] x 10° C x 100 grams) |
500 calories |
| To convert 100 grams of ice to water at -0° C requires: (80 cal/g [latent heat conversion] x 100 grams) |
8,000 calories |
| To raise 100 grams of water from 0° C to 100° C requires: (1.0 cal/g [specific heat of water] x 100° C x 100 grams) |
10,000 calories |
| To convert 100 grams of water to steam at 100° C requires: (540 cal/g [latent heat conversion] x 100 grams) |
54,000 calories |
| To raise 100 grams of steam at 100° C to 120° C requires: (0.5 cal/g [specific heat of steam] x 20° C x 100 grams) |
1,000 calories |
| Total energy required: | 73, 500 calories |


