3.5: Absolute Dating of Geologic Materials
- Page ID
- 21452
Numerical Ages
Numerical (also known as absolute) dating deals with assigning actual dates (in years before the present) to geological events. Contrast this with relative dating, which instead is concerned with determining the order of events in Earth’s past. The science of absolute age dating is known as geochronology and the fundamental method of geochronology is radiometric dating (also known as isotopic dating).
Absolute ages of rocks are determined from rates of radioactive decay of some isotopes of elements that occur naturally in rocks. These absolute ages might correspond to the crystallization age of a rock, the metamorphic age of a rock, or the time that has elapsed since a once-living organism died. There are a range of tools for these studies which will be discussed after a brief review of the chemistry associated with them.
Elements and Isotopes
An element is a particular kind of atom that is defined by the number of protons that it has in its nucleus. The number of protons equals the element’s atomic number. Have a look at the periodic table of the elements below. Carbon’s (C) atomic number is 6 because it has six protons in its nucleus; gold’s (Au) atomic number is 79 because it has 79 protons in its nucleus (Figure \(\PageIndex{1}\)).
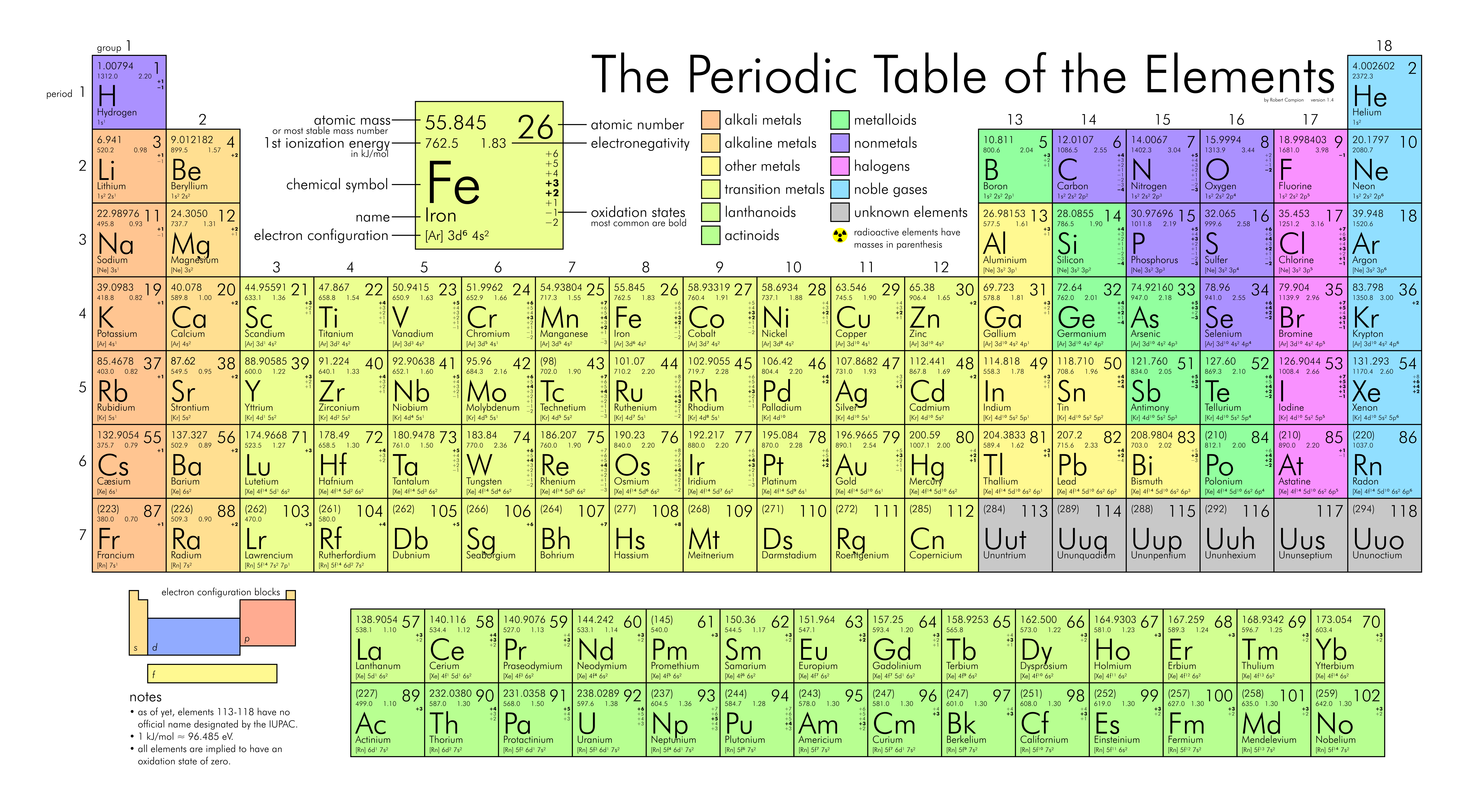
Even though individual elements always have the same number of protons, the number of neutrons in their nuclei sometimes varies. These variations are called isotopes. Isotopes of individual elements are defined by their mass number, which is simply the number of protons plus the number of neutrons.
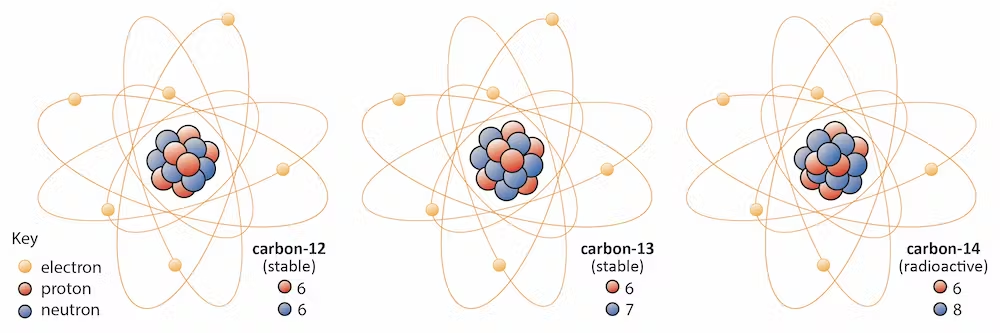
Consider, for example, the following three different isotopes of carbon as a demonstration of this concept:
-
Carbon-12 (12C): 6 protons, 6 neutrons
-
Carbon-13 (13C): 6 protons, 7 neutrons
-
Carbon-14 (14C): 6 protons, 8 neutrons
Figure \(\PageIndex{2}\) illustrates the arrangement of protons, neutrons and electrons in these carbon isotopes.
Radioactive Decay
While many common isotopes are stable, meaning that they do not change, or decay, over time, some isotopes are unstable and undergo radical change through the process of radioactive decay. When these isotopes, called “radioisotopes”, decay, they shed energy in the form of radiation and subatomic particles which causes changes to their numbers of protons and neutrons. Once the number of protons changes, the isotope is no longer the same element. Ultimately, radioisotopes will decay to form a different, stable element. The atomic nucleus that undergoes radioactive decay is known as the parent and the resulting stable element, the daughter product (or, decay product).
One cannot predict the radioactive decay of any individual atom, however because rock samples contain trillions of atoms, the overall pattern becomes statistically predictable. For example, in a hand sample of uranium ore, you don't know which individual uranium atom will decay next, but when you put a Geiger counter next to a sample you hear a continuous stream of radioactivity.
However, some rock specimens have an enormous number of radioactive isotopes, perhaps trillions of atoms, and this large group of radioactive isotopes does have a predictable pattern of radioactive decay. The radioactive decay of half of the radioactive isotopes in this group takes a specific amount of time. The time it takes for half of the atoms in a substance to decay is called the half-life. In other words, the half-life of an isotope is the amount of time it takes for half of a group of unstable isotopes to decay to a stable isotope. The half-life is constant and measurable for a given radioactive isotope, so it can be used to calculate the age of a rock.
Returning to the carbon example in the previous section, consider that there are 15 total isotopes of carbon. Most naturally occurring carbon is stable 12C (98.9%) or 13C (1.09%). The remainder of naturally occurring carbon, including 14C, is unstable and decays to form new elements. For example, carbon-14 (14C) decays to nitrogen-14 (14N); the half life of this decay is 5,730 years.
The principles behind numerical dating of geologic materials require three key assumptions.
-
The mineral grains containing the isotope formed at the same time as the rock, such as minerals in an igneous rock that crystallized from magma.
-
The mineral crystals remain a closed system, meaning they are not subsequently altered by elements moving in or out of them after they crystallize.
-
The only daughter isotopes present in the mineral crystal are created by radioactive decay of the parent (or there is a means to determine the original amount present).
These requirements place some constraints on the kinds of rock suitable for this type of dating, with igneous rock being the best. For example, igneous pyroclastic layers and lava flows within a sedimentary sequence can be used to date the sequence. Cross-cutting igneous rocks such as sills can be used to bracket the ages of affected, older sedimentary rocks. Igneous pyroclastic layers and lava flows within a sedimentary sequence can be used to date the sequence.
Metamorphic rocks are crystalline, but the processes of metamorphism may reset the clock and derived ages may represent a smear of different metamorphic events rather than the age of original crystallization.
Detrital sedimentary rocks are made from the pieces of older rocks and sometimes those pieces can be radiometrically dated; however, if we do that, then we learn the age of those pieces rather than when they came together into the sedimentary rock. In fact, the resistant mineral zircon, found as clasts in many ancient sedimentary rocks, has been successfully used for establishing very old dates, including the age of Earth’s oldest known rocks. Knowing that zircon minerals in metamorphosed sediments came from older rocks that are no longer available for study, scientists can date zircon to establish the age of the pre-metamorphic source rocks.
This short video reviews the concept of Radioactive Decay
Types of Radioactive Decay
There are several ways radioactive atoms decay; three that are most relevant for geologic applications are discussed below.
Alpha decay occurs when an alpha particle, which consists of two protons and two neutrons, is emitted from the nucleus of an atom (Figure \(\PageIndex{3}\)). This also happens to be the nucleus of a helium atom; helium gas may get trapped in the crystal lattice of a mineral in which alpha decay has taken place. When an atom loses two protons from its nucleus, lowering its atomic number, it is transformed into an element that is two atomic numbers lower on the Periodic Table of the Elements.
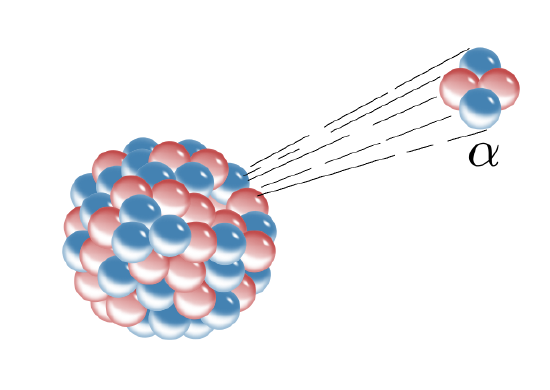
The loss of four particles, in this case two neutrons and two protons, also lowers the mass of the atom by four. For example alpha decay takes place in the unstable isotope 238U, which has an atomic number of 92 (92 protons) and mass number of 238 (total of all protons and neutrons). When 238U spontaneously emits an alpha particle, it becomes thorium-234 (234Th). The radioactive decay product of an element is called its daughter isotope and the original element is called the parent isotope. In this case, 238U is the parent isotope and 234Th is the daughter isotope. The half-life of 238U is 4.5 billion years, i.e., the time it takes for half of the parent isotope atoms to decay into the daughter isotope. This isotope of uranium, 238U, can be used for absolute dating the oldest materials found on Earth, and even meteorites and materials from the earliest events in our solar system.
Beta decay occurs when a neutron in its nucleus splits into an electron and a proton. The electron is emitted from the nucleus as a beta ray. The new proton increases the element’s atomic number by one, forming a new element with the same atomic mass as the parent isotope. For example, 234Th is unstable and undergoes beta decay to form protactinium-234 (234Pa), which also undergoes beta decay to form uranium-234 (234U). Notice these are all isotopes of different elements but they have the same atomic mass of 234. The decay process of radioactive elements like uranium keeps producing radioactive parents and daughters until a stable, or non-radioactive, daughter is formed. Such a series is called a decay chain. The decay chain of the radioactive parent isotope 238U is extensive and progresses through a series fourtenn different decay steps that include both alpha and beta decays and produce a range of different elements (some of which are extremely short-lived), until it forms the stable daughter isotope, lead-206 (206Pb).
Electron capture is when a proton in the nucleus captures an electron from one of the electron shells and becomes a neutron. This produces one of two different effects: 1) an electron jumps in to fill the missing spot of the departed electron and emits an X-ray, or 2) another electron is released and changes the atom into an ion. The atomic number is reduced by one and mass number remains the same. An example of an element that decays by electron capture is potassium-40 (40K). Radioactive 40K makes up a tiny percentage (0.012%) of naturally occurring potassium, most of which are not radioactive. 40K decays to argon-40 (40Ar) with a half-life of 1.25 billion years, so it is very useful for dating geological events. Below is a table of some of the more commonly-used radioactive dating isotopes and their half-lives.
|
Elements |
Parent symbol |
Daughter symbol |
Half-life |
|---|---|---|---|
|
Potassium-40/Argon-40* |
40K |
40Ar |
1.25 billion years |
|
Uranium-238/Lead-206 |
238U |
206Pb |
4.5 billion years |
|
Rubidium-87/Strontium-87 |
87Rb |
87Sr |
48.8 billion years |
|
Carbon-14/Nitrogen-14 |
14C |
14N |
5,730 years |
|
Uranium-235/Lead-207 |
235U |
207Pb |
704 million years |
*40Ar/40K is closely related to 40Ar/39Ar dating; both are in use today.
Radio-isotope Dating
For a given sample of rock the dating procedure begins with the chemical extraction of the parent and daughter isotopes from a mineral. Then the isotopes are measured; in the case of uranium, 238U and 235U isotopes are separated out together, as are the 206Pb and 207Pb with an instrument called a mass spectrometer.
Once the parent and daughter are measured, the age is calculated. The process of measuring the amount of parent and daughter and calculating an age can be quite complex and there are different considerations for each of the different systems listed on the table above.
For our purposes, we’ll consider a simple example in which one parent decays to form one daughter isotope. When the mineral initially forms, it consists of 0% daughter and 100% parent isotope, so the daughter-to-parent ratio (D/P) is 0. After one half-life, half the parent has decayed so there is 50% daughter and 50% parent, a 50/50 ratio, with D/P = 1. After two half-lives, there is 75% daughter and 25% parent (75/25 ratio) and D/P = 3. (Figure \(\PageIndex{4}\)).
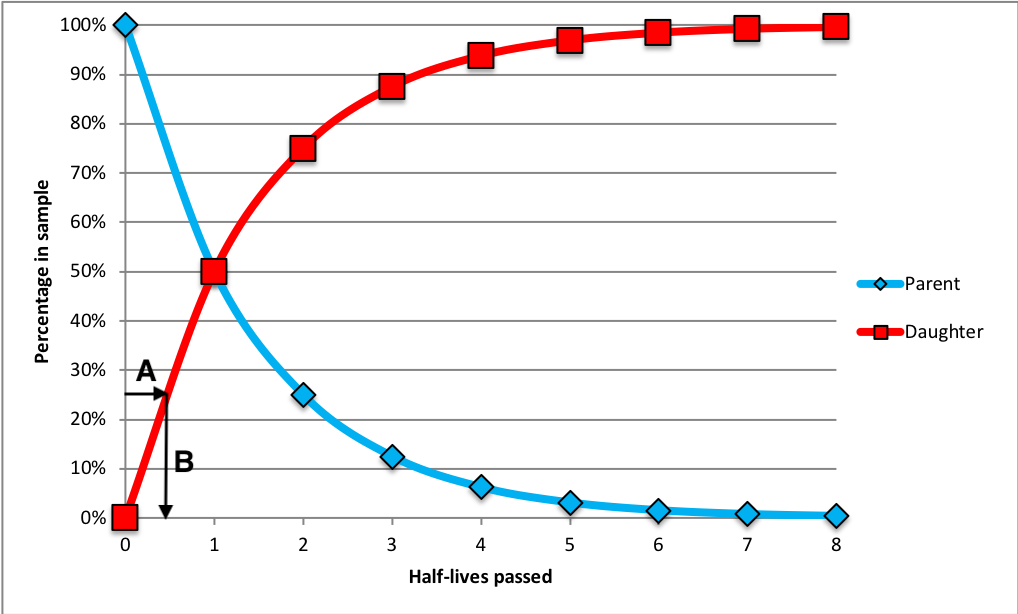
Modern applications of this method have achieved remarkable accuracies of plus or minus two million years in 2.5 billion years (that’s ± 0.055%). Applying the uranium/lead technique in any given sample analysis provides two separate clocks running at the same time, 238U and 235U. The existence of these two clocks in the same sample gives a cross-check between the two. Many geological samples contain multiple parent/daughter pairs, so cross-checking the clocks confirms that radioisotopic dating is highly reliable.
Another radioisotopic dating method involves carbon and is useful for dating archeologically important samples containing organic substances like wood or bone. Radiocarbon dating, also called carbon dating, uses the unstable isotope carbon-14 (14C) and the stable isotope carbon-12 (12C). Carbon-14 is constantly being created in the atmosphere by the interaction of cosmic particles with atmospheric nitrogen-14 (14N). Cosmic particles such as neutrons strike the nitrogen nucleus, kicking out a proton but leaving the neutron in the nucleus. The collision reduces the atomic number by one, changing it from seven to six, changing the nitrogen into carbon with the same mass number of 14. The 14C quickly bonds with oxygen (O) in the atmosphere to form carbon dioxide (14CO2) that mixes with other atmospheric carbon dioxide (12CO2) and this mix of gasses is incorporated into living matter. While an organism is alive, the ratio of 14C/12C in its body is in equilibrium with respect to the ratio in the environment because CO2 is constantly exchanged with the atmosphere. However, when the organism dies, the organism is no longer taking in carbon and excreting carbon, and the 4C/12C in its body gradually changes as the 14C decays back to 14N by beta decay. This process is the basis of this “clock”, which has a half-life of 5,730 years.
The radiocarbon dating technique relies upon the world's most sensitive weighing scale, the mass spectrometer, which can distinguish the mass of a single neutron. However, even a mass spectrometer has limits, and as time goes on, the radioactive parent isotopes diminish so much that there may be so little still remaining that we cannot meaningfully distinguish an experiment result from "background noise." Beyond about ten half lives--which for carbon dating is just over fifty thousand years--the remaining sample is too small to analyze. (Happily, for archeological investigations, fifty thousand years is perfectly in the range for dating human artifacts, but for the longer timescales of geology is not as useful.)
Query \(\PageIndex{1}\)
References
- Accessible Periodic Table of Elements. (n.d.). American Chemical Society. Retrieved August 14, 2023, from https://www.acs.org/education/students/highschool/olympiad/prepare-for-exams/accessible-periodic-table.html
- Bentley, C. (2020, January 17). Historical Geology – A free online textbook for Historical Geology courses. OpenGeology. Retrieved June 20, 2023, from https://opengeology.org/historicalgeology/
- Deline, B., Harris, R., & Tefend, K. (2016). Laboratory Manual for Introductory Geology. University of North Georgia.
- Earle, S. (2019). Physical Geology. BCcampus, BC Open Textbook Project.
- Johnson, C., Affolter, M. D., Inkenbrandt, P., & Mosher, C. (2019). An Introduction to Geology. Salt Lake Community College. https://slcc.pressbooks.pub/introgeology/
- Williams, E. (2013, January 10). Explainer: what is an isotope? The Conversation. Retrieved June 19, 2023, from https://theconversation.com/explainer-what-is-an-isotope-10688

