10.3: Identifying Sedimentary Rocks
- Page ID
- 5652
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Overview
The classification of sedimentary rocks is largely based on differentiating the processes that lead to their formation. The biggest division in types of sedimentary rocks types is based on the primary type of weathering that leads to the material building the sedimentary rock. If the rock is largely made from broken pieces (called clasts) of rock that have been mechanically weathered the rocks are referred to as Detrital or Clastic Sedimentary Rocks. Simply put, these are rocks that are composed of the broken pieces of other rocks. In this case, the mineralogy of the clasts is not important, but instead, we need to note the properties of the sediment itself. Alternatively, if the rock is largely the product of chemical weathering the classification is then based on the composition of the material as well as the processes involved in the materials precipitation from solution. Chemical Sedimentary Rocks form from the inorganic precipitation of minerals from a fluid. If the ions present within a fluid (water) become very concentrated either by the addition of more ions or the removal of water (by freezing or evaporation), then crystals begin to form. In this case, the identification of the type of sedimentary rocks is based on the minerals present. If organisms facilitate the precipitation of these minerals from water, we refer to the rocks as Biochemical Sedimentary Rocks. An example of biochemical precipitation is the formation of skeletal minerals in many organisms: from starfish and clams that grow calcite to sponges that grow silica-based material, to humans that have bones made of hydroxyapatite. In many cases, it is hard to differentiate whether a mineral was formed organically or inorganically, so in the current lab, we will mostly group these two types of sedimentary rocks together. Rocks can also be formed from the carbon-based organic material produced by ancient life and are called Organic Sedimentary Rocks. Now we can discuss the identification and formation of particular sedimentary rocks.
Clastic Sedimentary Rocks
Weathering and erosion occur normally in areas that are at high elevation, such as mountains, while deposition occurs in lower areas such as valleys, lakes, or the ocean. The sediment is transported from the area of erosion to area of deposition by ice, water, or air. Not surprisingly, the sediment changes during its journey and we can recognize the amount of change and the distance the material has traveled, and the transport mechanism, by looking at its maturity (Figure 10.1). Maturity is defined as the texture and composition of a sedimentary rock resulting from varying amounts of erosion or sedimentary transport. Imagine a mountain composed of the igneous rock granite and let us explore how the sediment from this mountain changes as it makes the long-distance trek via the river to the ocean. The first process is just breaking the rock down into smaller pieces mechanically, which creates sediment that has large and small pieces, the pieces are jagged, and all of the minerals remain. The sizes of clasts in these rocks can range from large boulders, to cobbles, to pebbles, to the smallest particles, clay. As this sediment is transported in the river the pebbles collide with other pebbles and the rocks get smaller and the sharp edges are broken off. Also, as the slope of the land decreases the river slows leaving behind the large boulders and cobbles while carrying away the smaller particles. This results in sediments further from the source to be more uniform in size, which is a process called sorting. Chemical weathering also occurs, altering the feldspars into clay-sized particles. The end result of this process is the granite reduced from boulders and cobbles close to the mountain, to pebbles in the rivers, and finally to pure and uniform quartz sand at the beach and minuscule clay grains on the ocean floor. Therefore, different clastic rocks are found in different areas and have traveled different distances.
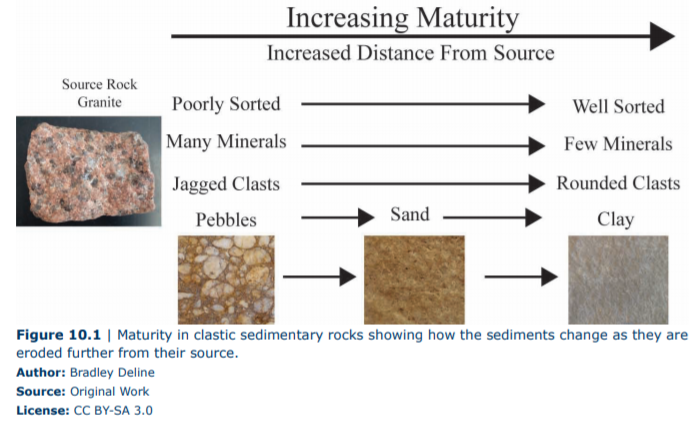
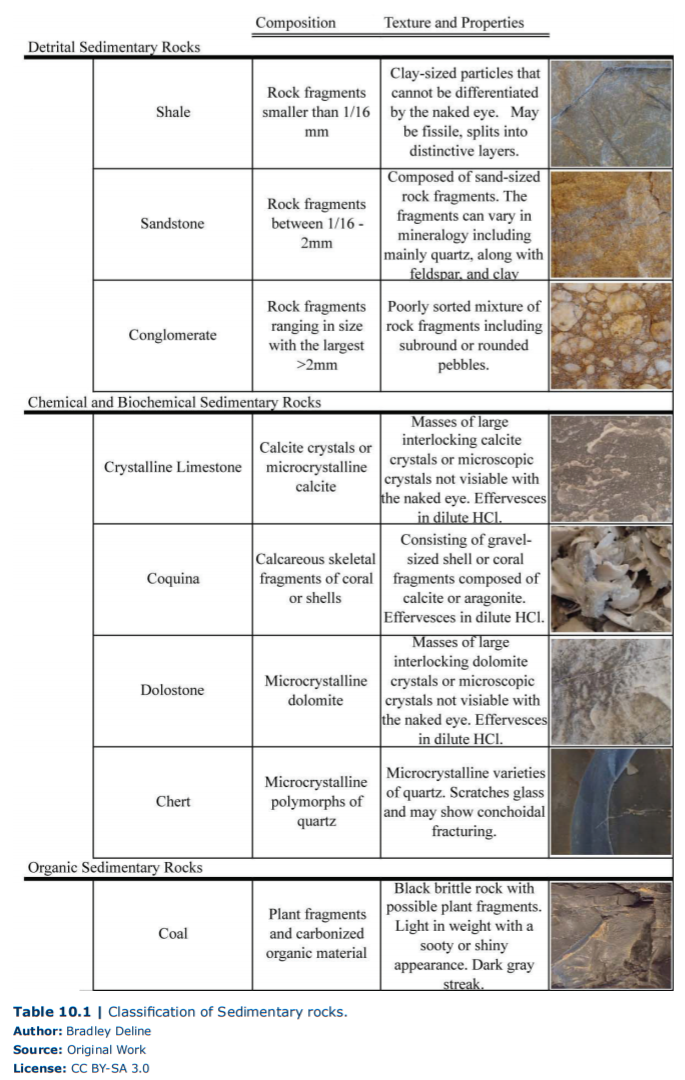
In this lab, we will look at three types of clastic rocks (Figure 10.1, Table 10.1), conglomerate, sandstone, and shale. Conglomerate is an immature sedimentary rock (rock that has been transported a short distance) that is a poorly sorted mixture of clay, sand, and rounded pebbles. The mineralogy of the sand and pebbles (also called clasts) can vary depending on its source. These rocks would be found on the continent in several types of deposits such as ancient landslides or pebble beds in rivers. Sandstone is defined as a clastic sedimentary rock that consists of sand-sized clasts. These clasts can vary from jagged to rounded as well as containing many minerals or just quartz. Therefore, sandstone ranges from being relatively immature to mature which makes sense because we can find layers of sand associated with mountain rivers to pure white quartz beaches. Last we have shale, which is composed of clay particles and has a finely layered or fissile appearance. This extremely mature sedimentary rock is made from the smallest particles that can be carried by wind or barely moving water and can be found thousands of miles away from the original source.
Biochemical and Chemical Sedimentary Rocks
As mentioned before, biochemical and chemical sedimentary rocks either precipitated directly from water or by organisms. The most recognizable chemical sedimentary rocks are evaporites. These are minerals that are formed by the precipitation of minerals from the evaporation of water. You have already examined multiple examples of these minerals/rocks in a previous lab, such as halite and gypsum. In this current lab, we will focus on siliceous and carbonate biochemical sedimentary rocks. Chert is a rock composed of microcrystalline varieties of quartz, and thus it has properties that are associated with quartz itself, such as conchoidal fracturing and hardness greater than glass. Chert is often formed deep in the ocean from siliceous material that is either inorganic (silica clay) or biologic (skeletons of sponges and single-celled organisms) in origin. Carbonates are one of the most important groups of sedimentary rocks and as you have previously learned (Chapter 5), can result in distinctive landscapes (karst) and human hazards (sinkholes). Limestone is a sedimentary rock composed of the carbonate mineral calcite and can vary greatly in its appearance depending on how it is formed, but can easily be identified by its chemical weathering. Limestone composed of the mineral calcite undergoes dissolution in acids. In other words, it effervesces dramatically when we apply dilute HCl. As with chert, limestone can be formed inorganically from the supersaturation of calcium and carbonate ions in water in varying environments from caves to tropical beaches. Limestone that consists of crystals of calcite or microcrystalline masses of calcite is called crystalline limestone. Alternatively, limestone can be formed biologically with the most striking example called coquina, which are rocks made exclusively of fragmented carbonate (calcite or its polymorph aragonite) shells or coral. Lastly, we have dolostones, which are made from crystals (large or microscopic) of the mineral dolomite and are a carbonate that only weakly reacts to dilute HCl; you can scratch and powder dolostone to increase the surface area to see the reaction with acid. Dolostone is formed by the inorganic chemical alteration of limestones, therefore they are classified as chemical rather than biochemical sedimentary rocks.
Organic Sedimentary Rocks
Organic compounds are materials that contain a significant amount of the element carbon and are often associated with life. Organic sedimentary rocks are, therefore, rocks that consist mostly of carbon and are associated with significant biological activity. Other sedimentary rocks such as limestone and shale can contain carbon, but at much lower concentration (though shale can appear black from their carbon content). The most common organic sedimentary rock is coal, which is a very low density (light) black rock that has a dusty (sooty) or shiny appearance. It also produces a dark gray streak that can be seen both on a streak plate or a piece of paper. Coal is formed from the preservation and compaction of abundant plant material often in areas where oxygen is lacking, such as a swamp.


