8.2: Igneous Rock Origin
- Page ID
- 5593
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Magma Composition
It seems like a bad joke, but before any igneous rock can form, there must be molten material known as magma produced, which means that you must first have a rock to melt to make magma in order for it to cool and become an igneous rock. Which brings more questions: what rock melted to form the magma? Was there more than one rock type that melted to form that magma? Did the rocks completely melt, or did only certain minerals inside of those rocks melt (a process known as partial melting)? Once that melted material formed, what happened to it next? Did some other process occur to change the composition of that magma, before ending up as the igneous rock that we are studying? These are just a few of the questions that a person should consider when studying the origin of igneous rocks.
Most rocks (there are very few exceptions!) contain minerals that are crystalline solids composed of the chemical elements. In your chapter on minerals, you learned that the most common minerals belong to a group known as the silicate minerals, so it makes sense that magmas form from the melting of rocks that most likely contain abundant silicate minerals. However, all minerals (not just the silicates) have a certain set of conditions, such as temperature, at which they can melt. Since rocks contain a mixture of minerals, it is easy to see how only some of the minerals in a rock may melt and why others stay as a solid. Furthermore, the temperature conditions are important (as only minerals that can melt at “lower” temperatures (such as 600°C) may experience melting) whereas the temperature would have to increase (for example, to 1200°C) in order for other minerals to also melt (remember the lower temperature minerals are still melting) and thus add their chemical components to the magma that is being generated. This brings up an important point: even if the same types of rocks are melting, we can generate different magma compositions purely by melting at different temperatures!
Once magma is generated, it will eventually start to rise upward through the Earth’s lithosphere, as magma is more buoyant than the source rock that generated it. This separation of the magma from the source region will result in new thermal conditions as the magma moves away from the heated portion of the lithosphere and encounters cooler rocks, which results in the magma also cooling. As with melting, minerals also have a certain set of conditions at which they form, or crystallize, from within a cooling magma body. You would be right in thinking that the sequence of mineral crystallization is the opposite sequence of crystal melting. The sequence of mineral formation from magma has been experimentally determined by Norman L. Bowen in the early 1900s, and the now famous Bowen’s reaction series appears in countless textbooks and lab manuals (Figure 8.1).
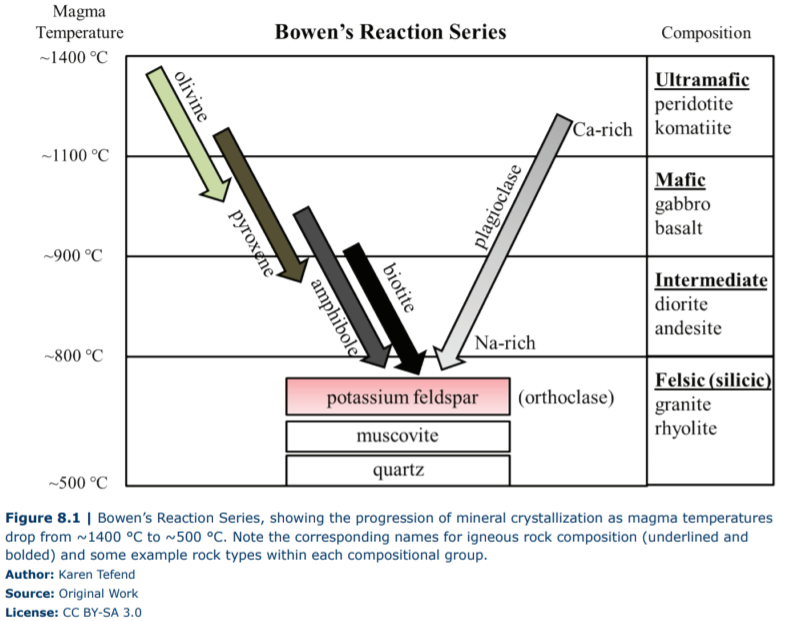
This “reaction series” refers to the chemical reactions that are the formation of minerals, through the chemical bonding of elements within the magma, in a sequence that is based on falling magma temperatures. Close examination of Figure 8.1 shows that the first mineral to crystallize in a cooling magma of ultramafic composition is olivine; the length of the arrow indicates the range of temperatures at which olivine can form. Once temperatures fall below this range, olivine crystals will no longer form; instead, other minerals such as pyroxene will start to crystallize (a small interval of temperatures exists where both olivine and pyroxene can crystallize). Minerals that form in cooling magma are called crystals or phenocrysts. As these phenocrysts are forming, they are removing chemical elements from the magma. For example, olivine phenocrysts take magnesium (Mg) and iron (Fe) from the magma and incorporate them into their crystal structure. This behavior of mineral phenocrysts to take certain chemical elements into their structure, while excluding other elements, means that the composition of the magma must be changing as phenocrysts are forming!
There can be more than one mineral type crystallizing within the cooling magma, as the arrows in Figure 8.1 demonstrate. The minerals on the left side of Bowen’s reaction series are referred to as a discontinuous series, as these minerals (olivine, pyroxene, amphibole, and biotite) all remove the iron (Fe), magnesium (Mg), and manganese (Mn) from the magma during crystallization, but do so at certain temperature ranges. These iron- and magnesium-rich minerals are referred to as ferromagnesian minerals (ferro = iron) and are usually green, dark gray, or black in color due to the absorption of visible light by iron and magnesium atoms. On the right side of Bowen’s reaction series is a long arrow labeled plagioclase feldspar. Plagioclase crystallizes over a large temperature interval and represents a continuous series of crystallization even though its composition changes from calcium (Ca) rich to sodium (Na) rich. As the magma temperature drops and plagioclase first starts to crystallize (form), it will take in the calcium atoms into the crystal structure, but as magma temperatures continue to drop, plagioclase takes in sodium atoms preferentially. As a result, the higher temperature calcium-rich plagioclase is dark gray in color due to the high calcium content, but the lower temperature sodium-rich plagioclase is white due to the high sodium content. Finally, at the bottom of the graph in Figure 8.1, we see that three more minerals can form as temperatures continue to drop. These minerals (potassium feldspar, muscovite, and quartz) are considered to be the “low-temperature minerals”, as they are the last to form during cooling, and therefore first to melt as a rock is heated. The previous removal of iron and magnesium from the magma results in the formation of the latest-forming minerals that are deficient in these chemical elements; these minerals are referred to as nonferromagnesian minerals, which are much lighter in color. For example, the potassium-rich feldspar (also known as orthoclase) can be a pale pink or white in color. The references to mineral color are necessary, as the color of any mineral is primarily due to the chemical elements that are in the minerals, and therefore the color of igneous rocks will be dependent on the mineral content (or chemical composition) of the rock.


