11.4: Climate Change
- Page ID
- 11794
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Causes of Climate Change
Earth’s Temperature is a Balancing Act
Earth’s temperature depends on the balance between energy entering and leaving the planet’s system . When incoming energy from the sun is absorbed by the Earth system, Earth warms. When the sun’s energy is reflected back into space, Earth avoids warming. When energy is released back into space, Earth cools. Many factors, both natural and human, can cause changes in Earth’s energy balance, including:
- Changes in the greenhouse effect, which affects the amount of heat retained by Earth’s atmosphere
- Variations in the sun’s energy reaching Earth
- Changes in the reflectivity of Earth’s atmosphere and surface
These factors have caused Earth’s climate to change many times.
Scientists have pieced together a picture of Earth’s climate, dating back hundreds of thousands of years, by analyzing a number of indirect measures of climate such as ice cores, tree rings, glacier lengths, pollen remains, and ocean sediments, and by studying changes in Earth’s orbit around the sun.
The historical record shows that the climate system varies naturally over a wide range of time scales. In general, climate changes prior to the Industrial Revolution in the 1700s can be explained by natural causes, such as changes in solar energy, volcanic eruptions, and natural changes in greenhouse gas (GHG) concentrations.
Recent climate changes, however, cannot be explained by natural causes alone. Research indicates that natural causes are very unlikely to explain most observed warming, especially warming since the mid-20th century. Rather, human activities can very likely explain most of that warming.
The greenhouse effect causes the atmosphere to retain heat
When sunlight reaches Earth’s surface, it can either be reflected back into space or absorbed by Earth. Once absorbed, the planet releases some of the energy back into the atmosphere as heat (also called infrared radiation). Greenhouse gases (GHGs) like water vapor (\(\ce{H2O}\)), carbon dioxide (\(\ce{CO2}\)), and methane (\(\ce{CH4}\)) absorb energy, slowing or preventing the loss of heat to space. In this way, GHGs act like a blanket, making Earth warmer than it would otherwise be. This process is commonly known as the “greenhouse effect”.
What is Global Warming?
Global warming refers to the recent and ongoing rise in global average temperature near Earth's surface. It is caused mostly by increasing concentrations of greenhouse gases in the atmosphere. Global warming is causing climate patterns to change. However, global warming itself represents only one aspect of climate change.
What is Climate Change?
Climate change refers to any significant change in the measures of climate lasting for an extended period of time. In other words, climate change includes major changes in temperature, precipitation, or wind patterns, among other effects, that occur over several decades or longer.
Humans are largely responsible for recent climate change
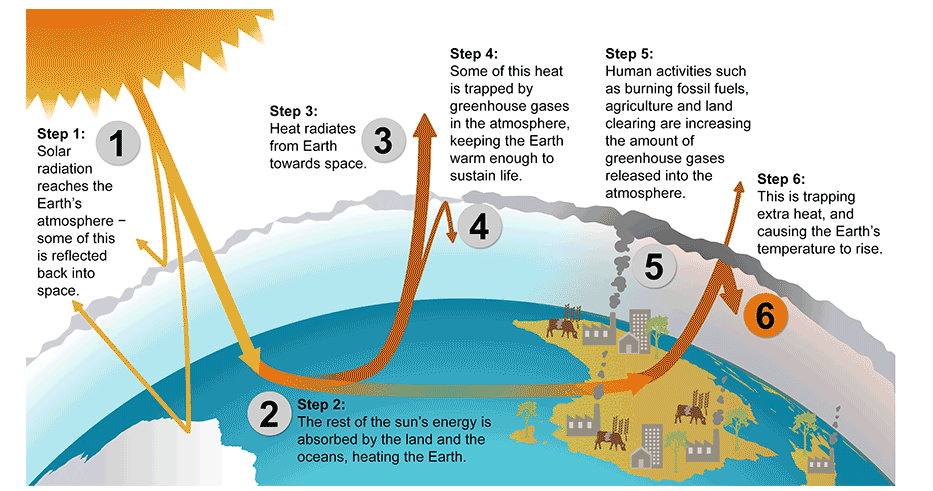 Figure \(\PageIndex{1}\): Enhanced Greenhouse Effect
Figure \(\PageIndex{1}\): Enhanced Greenhouse Effect
The Main Greenhouse Gasses
The most important GHGs directly emitted by humans include \(\ce{CO2}\), \(\ce{CH}\), nitrous oxide (\(\ce{CN2O}\)), and several others. The sources and recent trends of these gases are detailed below.
Carbon dioxide. Carbon dioxide (\(\ce{CO2}\)) is the primary greenhouse gas that is contributing to recent climate change. \(\ce{CO2}\) is absorbed and emitted naturally as part of the carbon cycle, through animal and plant respiration, volcanic eruptions, and ocean-atmosphere exchange. Human activities, such as the burning of fossil fuels and changes in land use, release large amounts of carbon to the atmosphere, causing \(\ce{CO2}\) concentrations in the atmosphere to rise.
Atmospheric \(\ce{CO2}\) concentrations have increased by almost 40% since pre-industrial times, from approximately 280 parts per million by volume (ppmv) in the 18th century to 390 ppmv in 2010. The current \(\ce{CO2}\) level is higher than it has been in at least 800,000 years.Some volcanic eruptions released large quantities of \(\ce{CO2}\) in the distant past. However, the U.S. Geological Survey (USGS) reports that human activities now emit more than 135 times as much \(\ce{CO2}\) as volcanoes each year. Human activities currently release over 30 billion tons of \(\ce{CO2}\) into the atmosphere every year.This build-up in the atmosphere is like a tub filling with water, where more water flows from the faucet than the drain can take away.
Methane. Methane (\(\ce{CH4}\)) is produced through both natural and human activities. For example, natural wetlands, agricultural activities, and fossil fuel extraction and transport all emit \(\ce{CH4}\).
Methane is more abundant in Earth’s atmosphere now than at any time in at least the past 650,000 years.Due to human activities, CH4concentrations increased sharply during most of the 20th century and are now more than two and-a-half times pre-industrial levels. In recent decades, the rate of increase has slowed considerably.
Nitrous oxide. Nitrous oxide (\(\ce{N2O}\)) is produced through natural and human activities, mainly through agricultural activities and natural biological processes. Fuel burning and some other processes also create \(\ce{N2O}\). Concentrations of \(\ce{N2O}\) have risen approximately 18% since the start of the Industrial Revolution, with a relatively rapid increase towards the end of the 20th century.In contrast, the atmospheric concentration of \(\ce{N2O}\) varied only slightly for a period of 11,500 years before the onset of the industrial period, as shown by ice core samples.
Other Greenhouse Gasses
Water vapor is the most abundant greenhouse gas and also the most important in terms of its contribution to the natural greenhouse effect, despite having a short atmospheric lifetime. Some human activities can influence local water vapor levels. However, on a global scale, the concentration of water vapor is controlled by temperature, which influences overall rates of evaporation and precipitation.Therefore, the global concentration of water vapor is not substantially affected by direct human emissions.
Tropospheric ozone (O3), which also has a short atmospheric lifetime, is a potent greenhouse gas. Chemical reactions create ozone from emissions of nitrogen oxides and volatile organic compounds from automobiles, power plants, and other industrial and commercial sources in the presence of sunlight. In addition to trapping heat, ozone is a pollutant that can cause respiratory health problems and damage crops and ecosystems.
Chlorofluorocarbons (CFCs), hydrochlorofluorocarbons (HCFCs), hydrofluorocarbons (HFCs), perfluorocarbons (PFCs), and sulfur hexafluoride (SF6), together called F-gases, are often used in coolants, foaming agents, fire extinguishers, solvents, pesticides, and aerosol propellants. Unlike water vapor and ozone, these F-gases have a long atmospheric lifetime, and some of these emissions will affect the climate for many decades or centuries.
Changes in the sun’s energy affect how much energy reaches Earth’s system
Climate is influenced by natural changes that affect how much solar energy reaches Earth. These changes include changes within the sun and changes in Earth’s orbit. Changes occurring in the sun itself can affect the intensity of the sunlight that reaches Earth’s surface. The intensity of the sunlight can cause either warming (during periods of stronger solar intensity) or cooling (during periods of weaker solar intensity). The sun follows a natural 11-year cycle of small ups and downs in intensity, but the effect on Earth’s climate is small.Changes in the shape of Earth’s orbit as well as the tilt and position of Earth’s axis can also affect the amount of sunlight reaching Earth’s surface.
Changes in the sun’s intensity have influenced Earth’s climate in the past. For example, the so-called “Little Ice Age” between the 17th and 19th centuries may have been partially caused by a low solar activity phase from 1645 to 1715, which coincided with cooler temperatures. The “Little Ice Age” refers to a slight cooling of North America, Europe, and probably other areas around the globe.
Changes in Earth’s orbit have had a big impact on climate over tens of thousands of years. In fact, the amount of summer sunshine on the Northern Hemisphere, which is affected by changes in the planet’s orbit, appears to control the advance and retreat of ice sheets. These changes appear to be the primary cause of past cycles of ice ages, in which Earth has experienced long periods of cold temperatures (ice ages), as well as shorter interglacial periods (periods between ice ages) of relatively warmer temperatures.
Changes in solar energy continue to affect climate. However, solar activity has been relatively constant, aside from the 11-year cycle, since the mid-20th century and therefore does not explain the recent warming of Earth. Similarly, changes in the shape of Earth’s orbit as well as the tilt and position of Earth’s axis affect temperature on relatively long timescales (tens of thousands of years), and therefore cannot explain the recent warming.
Changes in reflectivity affect how much energy enters Earth’s system
When sunlight reaches Earth, it can be reflected or absorbed. The amount that is reflected or absorbed depends on Earth’s surface and atmosphere. Light-colored objects and surfaces, like snow and clouds, tend to reflect most sunlight, while darker objects and surfaces, like the ocean, forests, or soil, tend to absorb more sunlight.
The term albedo refers to the amount of solar radiation reflected from an object or surface, often expressed as a percentage. Earth as a whole has an albedo of about 30%, meaning that 70% of the sunlight that reaches the planet is absorbed. Absorbed sunlight warms Earth’s land, water, and atmosphere.
Reflectivity is also affected by aerosols. Aerosols are small particles or liquid droplets in the atmosphere that can absorb or reflect sunlight. Unlike greenhouse gases (GHGs), the climate effects of aerosols vary depending on what they are made of and where they are emitted. Those aerosols that reflect sunlight, such as particles from volcanic eruptions or sulfur emissions from burning coal, have a cooling effect. Those that absorb sunlight, such as black carbon (a part of soot), have a warming effect.
The Role of Reflectivity in the Past
Natural changes in reflectivity, like the melting of sea ice or increases in cloud cover, have contributed to climate change in the past, often acting as feedbacks to other processes.
Volcanoes have played a noticeable role in climate. Volcanic particles that reach the upper atmosphere can reflect enough sunlight back to space to cool the surface of the planet by a few tenths of a degree for several years.These particles are an example of cooling aerosols. Volcanic particles from a single eruption do not produce long-term change because they remain in the atmosphere for a much shorter time than GHGs.
The Recent Role of Reflectivity
Human changes in land use and land cover have changed Earth’s reflectivity. Processes such as deforestation, reforestation, desertification, and urbanization often contribute to changes in climate in the places they occur. These effects may be significant regionally, but are smaller when averaged over the entire globe.
In addition, human activities have generally increased the number of aerosol particles in the atmosphere. Overall, human-generated aerosols have a net cooling effect offsetting about one-third of the total warming effect associated with human greenhouse gas emissions. Reductions in overall aerosol emissions can therefore lead to more warming. However, targeted reductions in black carbon emissions can reduce warming.
Is there a scientific consensus on climate change?
The major scientific agencies of the United States — including the National Aeronautics and Space Administration (NASA) and the National Oceanic and Atmospheric Administration (NOAA) — agree that climate change is occurring and that humans are contributing to it. In 2010, the National Research Council concluded that "Climate change is occurring, is very likely caused by human activities, and poses significant risks for a broad range of human and natural systems". Many independent scientific organizations have released similar statements, both in the United States and abroad. This doesn't necessarily mean that every scientist sees eye to eye on each component of the climate change problem, but broad agreement exists that climate change is happening and is primarily caused by excess greenhouse gases from human activities.
Future Climate Change
Increasing greenhouse gas concentrations will have many effects
Greenhouse gas concentrations in the atmosphere will continue to increase unless the billions of tons of our annual emissions decrease substantially. Increased concentrations are expected to:
- Increase Earth's average temperature
- Influence the patterns and amounts of precipitation
- Reduce ice and snow cover, as well as permafrost
- Raise sea level
- Increase the acidity of the oceans
These changes will impact our food supply, water resources, infrastructure, ecosystems, and even our own health
Future changes will depend on many factors
The magnitude and rate of future climate change will primarily depend on the following factors:
- The rate at which levels of greenhouse gas concentrations in our atmosphere continue to increase
- How strongly features of the climate (e.g., temperature, precipitation, and sea level) respond to the expected increase in greenhouse gas concentrations
- Natural influences on climate (e.g., from volcanic activity and changes in the sun's intensity) and natural processes within the climate system (e.g., changes in ocean circulation patterns)
Past and present-day greenhouse gas emissions will affect climate far into the future
Many greenhouse gases stay in the atmosphere for long periods of time. As a result, even if emissions stopped increasing, atmospheric greenhouse gas concentrations would continue to increase and remain elevated for hundreds of years. Moreover, if we stabilized concentrations and the composition of today's atmosphere remained steady (which would require a dramatic reduction in current greenhouse gas emissions), surface air temperatures would continue to warm. This is because the oceans, which store heat, take many decades to fully respond to higher greenhouse gas concentrations. The ocean's response to higher greenhouse gas concentrations and higher temperatures will continue to impact climate over the next several decades to hundreds of years.
Because it is difficult to project far-off future emissions and other human factors that influence climate, scientists use a range of scenarios using various assumptions about future economic, social, technological, and environmental conditions.
 Figure \(\PageIndex{1}\) shows projected greenhouse gas concentrations for four different emissions scenarios. The top three scenarios assume no explicit climate policies. The bottom green line is an illustrative “stabilization scenario,” designed to stabilize atmospheric carbon dioxide concentration at 450 parts per million by volume (ppmv). Source: USGCRP (2009)
Figure \(\PageIndex{1}\) shows projected greenhouse gas concentrations for four different emissions scenarios. The top three scenarios assume no explicit climate policies. The bottom green line is an illustrative “stabilization scenario,” designed to stabilize atmospheric carbon dioxide concentration at 450 parts per million by volume (ppmv). Source: USGCRP (2009)
Future Temperature Changes
We have already observed global warming over the last several decades. Future temperatures are expected to change further. Climate models project the following key temperature-related changes.
Key Global Projections
- Average global temperatures are expected to increase by 2°F to 11.5°F by 2100, depending on the level of future greenhouse gas emissions, and the outcomes from various climate models.
- By 2100, global average temperature is expected to warm at least twice as much as it has during the last 100 years.
- Ground-level air temperatures are expected to continue to warm more rapidly over land than oceans.
- Some parts of the world are projected to see larger temperature increases than the global average.
 Projected changes in global average temperatures under three emissions scenarios (rows) for three different time periods (columns). Changes in temperatures are relative to 1961-1990 averages. The scenarios come from the IPCC Special Report on Emissions Scenarios: B1 is a low emissions scenario, A1B is a medium-high emissions scenario, and A2 is a high emissions scenario. Source: NRC 2010
Projected changes in global average temperatures under three emissions scenarios (rows) for three different time periods (columns). Changes in temperatures are relative to 1961-1990 averages. The scenarios come from the IPCC Special Report on Emissions Scenarios: B1 is a low emissions scenario, A1B is a medium-high emissions scenario, and A2 is a high emissions scenario. Source: NRC 2010
Observed and projected changes in global average temperature under three no-policy emissions scenarios. The shaded areas show the likely ranges while the lines show the central projections from a set of climate models. A wider range of model types shows outcomes from 2 to 11.5°F. Changes are relative to the 1960-1979 average. Source: USGCRP 2009
Future Precipitation and Storm Events
Patterns of precipitation and storm events, including both rain and snowfall are also likely to change. However, some of these changes are less certain than the changes associated with temperature. Projections show that future precipitation and storm changes will vary by season and region. Some regions may have less precipitation, some may have more precipitation, and some may have little or no change. The amount of rain falling in heavy precipitation events is likely to increase in most regions, while storm tracks are projected to shift poleward. Climate models project the following precipitation and storm changes
Key Global Projections
- Global average annual precipitation through the end of the century is expected to increase, although changes in the amount and intensity of precipitation will vary by region.
- The intensity of precipitation events will likely increase on average. This will be particularly pronounced in tropical and high-latitude regions, which are also expected to experience overall increases in precipitation.
- The strength of the winds associated with tropical storms is likely to increase. The amount of precipitation falling in tropical storms is also likely to increase.
- Annual average precipitation is projected to increase in some areas and decrease in others. The figure to the right shows projected regional differences in precipitation for summer and winter.
Future Ice, Snowpack, and Permafrost
Arctic sea ice is already declining.The area of snow cover in the Northern Hemisphere has decreased since about 1970. [7]Permafrost temperature has increased over the last century.These are just three of the many forms of snow and ice found on Earth. To learn more about the different forms of snow and ice and how they affect the global climate system, visit the Snow and Ice page of the Indicators section. Over the next century, it is expected that sea ice will continue to decline, glaciers will continue to shrink, snow cover will continue to decrease, and permafrost will continue to thaw. Potential changes to ice, snow, and permafrost are described below.
Key Global Projections
For every 2°F of warming, models project about a 15% decrease in the extent of annually averaged sea ice and a 25% decrease in September Arctic sea ice. The coastal sections of the Greenland and Antarctic ice sheets are expected to continue to melt or slide into the ocean. If the rate of this ice melting increases in the 21st century, the ice sheets could add significantly to global sea level rise. Glaciers are expected to continue to decrease in size. The rate of melting is expected to continue to increase, which will contribute to sea level rise.
Future Sea Level Change
Warming temperatures contribute to sea level rise by: expanding ocean water; melting mountain glaciers and ice caps; and causing portions of the Greenland and Antarctic ice sheets to melt or flow into the ocean. Since 1870, global sea level has risen by about 8 inches. Estimates of future sea level rise vary for different regions, but global sea level for the next century is expected to rise at a greater rate than during the past 50 years. The contribution of thermal expansion, ice caps, and small glaciers to sea level rise is relatively well-studied, but the impacts of climate change on ice sheets are less understood and represent an active area of research. Thus it is more difficult to predict how much changes in ice sheets will contribute to sea level rise. Greenland and Antarctic ice sheets could contribute an additional 1 foot of sea level rise, depending on how the ice sheets respond.
Regional and local factors will influence future relative sea level rise for specific coastlines around the world. For example, relative sea level rise depends on land elevation changes that occur as a result of subsidence (sinking) or uplift (rising). Assuming that these historical geological forces continue, a 2-foot rise in global sea level by 2100 would result in the following relative sea level rise:
- 2.3 feet at New York City
- 2.9 feet at Hampton Roads, Virginia
- 3.5 feet at Galveston, Texas
- 1 foot at Neah Bay in Washington state
Relative sea level rise also depends on local changes in currents, winds, salinity, and water temperatures, as well as proximity to thinning ice sheets.
Future Ocean Acidification
Corals require the right combination of temperature, light, and the presence of calcium carbonate (which they use to build their skeletons). As atmospheric carbon dioxide (\(\ce{CO2}\)) levels rise, some of the excess \(\ce{CO2}\) dissolves into ocean water, reducing its calcium carbonate saturation. As the maps indicate, calcium carbonate saturation has already been reduced considerably from its pre-industrial level, and model projections suggest much greater reductions in the future. The blue dots indicate current coral reefs. Note that under projections for the future, it is very unlikely that calcium carbonate saturation levels will be adequate to support coral reefs in any U.S. waters.
Oceans become more acidic as carbon dioxide (\(\ce{CO2}\)) emissions in the atmosphere dissolve in the ocean. This change is measured on the pH scale, with lower values being more acidic. The pH level of the oceans has decreased by approximately 0.1 pH units since pre-industrial times, which is equivalent to a 25% increase in acidity. The pH level of the oceans is projected to decrease even more by the end of the century as \(\ce{CO2}\) concentrations are expected to increase for the foreseeable future. Ocean acidification adversely affects many marine species, including plankton, mollusks, shellfish, and corals. As ocean acidification increases, the availability of calcium carbonate will decline. Calcium carbonate is a key building block for the shells and skeletons of many marine organisms. If atmospheric \(\ce{CO2}\) concentrations double, coral calcification rates are projected to decline by more than 30%. If \(\ce{CO2}\) concentrations continue to rise at their current rate, corals could become rare on tropical and subtropical reefs by 2050.
Climate change affects everyone
Our lives are connected to the climate. Human societies have adapted to the relatively stable climate we have enjoyed since the last ice age which ended several thousand years ago. A warming climate will bring changes that can affect our water supplies, agriculture, power and transportation systems, the natural environment, and even our own health and safety.
Some changes to the climate are unavoidable. Carbon dioxide can stay in the atmosphere for nearly a century, so Earth will continue to warm in the coming decades. The warmer it gets, the greater the risk for more severe changes to the climate and Earth's system. Although it's difficult to predict the exact impacts of climate change, what's clear is that the climate we are accustomed to is no longer a reliable guide for what to expect in the future.
We can reduce the risks we will face from climate change. By making choices that reduce greenhouse gas pollution, and preparing for the changes that are already underway, we can reduce risks from climate change. Our decisions today will shape the world our children and grandchildren will live in.
You Can Take Action
You can take steps at home, on the road, and in your office to reduce greenhouse gas emissions and the risks associated with climate change. Many of these steps can save you money; some, such as walking or biking to work can even improve your health! You can also get involved on a local or state level to support energy efficiency, clean energy programs, or other climate programs.
Learn more about what you can do.
Calculate your carbon footprint and find ways to reduce your emissions through simple everyday actions.

