4.7: Igneous Rocks
- Page ID
- 12788
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Igneous rocks form from the cooling and hardening of molten magma in many different environments. These rocks are identified by their composition and texture. More than 700 different types of igneous rocks are known.
Magma Composition
 The rock beneath the Earth’s surface is sometimes heated to high enough temperatures that it melts to create magma. Different magmas have different composition and contain whatever elements were in the rock that melted. Magmas also contain gases. The main elements are the same as the elements found in the crust. Whether rock melts to create magma depends on:
The rock beneath the Earth’s surface is sometimes heated to high enough temperatures that it melts to create magma. Different magmas have different composition and contain whatever elements were in the rock that melted. Magmas also contain gases. The main elements are the same as the elements found in the crust. Whether rock melts to create magma depends on:
- Temperature: Temperature increases with depth, so melting is more likely to occur at greater depths.
- Pressure: Pressure increases with depth, but increased pressure raises the melting temperature, so melting is less likely to occur at higher pressures.
- Water: The addition of water changes the melting point of rock. As the amount of water increases, the melting point decreases.
- Rock composition: Minerals melt at different temperatures, so the temperature must be high enough to melt at least some minerals in the rock. The first mineral to melt from a rock will be quartz (if present) and the last will be olivine (if present).
The different geologic settings that produce varying conditions under which rocks melt will be discussed in the “Plate Tectonics” chapter. As a rock heats up, the minerals that melt at the lowest temperatures will melt first. Partial melting occurs when the temperature on a rock is high enough to melt only some of the minerals in the rock. The minerals that will melt will be those that melt at lower temperatures. Fractional crystallization is the opposite of partial melting. This process describes the crystallization of different minerals as magma cools.
Bowen’s Reaction Series indicates the temperatures at which minerals melt or crystallize. An understanding of the way atoms join together to form minerals leads to an understanding of how different igneous rocks form. Bowen’s Reaction Series also explains why some minerals are always found together and some are never found together.
If the liquid separates from the solids at any time in partial melting or fractional crystallization, the chemical composition of the liquid and solid will be different. When that liquid crystallizes, the resulting igneous rock will have a different composition from the parent rock.
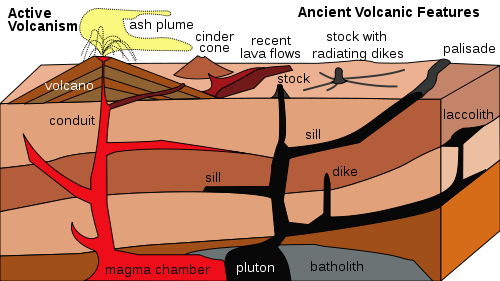
Intrustive Igneous Rock
 Igneous rocks are called intrusive when they cool and solidify beneath the surface. Intrusive rocks form plutons and so are also called plutonic. A pluton is an igneous intrusive rock body that has cooled in the crust. When magma cools within the Earth, the cooling proceeds slowly. Slow cooling allows time for large crystals to form, so intrusive igneous rocks have visible crystals. Granite is the most common intrusive igneous rock.Igneous rocks make up most of the rocks on Earth. Most igneous rocks are buried below the surface and covered with sedimentary rock, or are buried beneath the ocean water. In some places, geological processes have brought igneous rocks to the surface. Yosemite is a classic example of intrusive igneous rock. The molten magma never reached Earth’s surface, so the molten material had millions of years to cool down slowly to form granite. Later, geologic forces and erosion have caused those granite plutons to surface as they are today.
Igneous rocks are called intrusive when they cool and solidify beneath the surface. Intrusive rocks form plutons and so are also called plutonic. A pluton is an igneous intrusive rock body that has cooled in the crust. When magma cools within the Earth, the cooling proceeds slowly. Slow cooling allows time for large crystals to form, so intrusive igneous rocks have visible crystals. Granite is the most common intrusive igneous rock.Igneous rocks make up most of the rocks on Earth. Most igneous rocks are buried below the surface and covered with sedimentary rock, or are buried beneath the ocean water. In some places, geological processes have brought igneous rocks to the surface. Yosemite is a classic example of intrusive igneous rock. The molten magma never reached Earth’s surface, so the molten material had millions of years to cool down slowly to form granite. Later, geologic forces and erosion have caused those granite plutons to surface as they are today.
Extrusive Igneous Rock
 Igneous rocks are called extrusive when they cool and solidify above the surface. These rocks usually form from a volcano, so they are also called volcanic rocks. Extrusive igneous rocks cool much more rapidly than intrusive rocks. There is little time for crystals to form, so extrusive igneous rocks have tiny crystals.Cooling rate and gas content create a variety of rock textures. Lavas that cool extremely rapidly may have a glassy texture. Those with many holes from gas bubbles have a vesicular texture.
Igneous rocks are called extrusive when they cool and solidify above the surface. These rocks usually form from a volcano, so they are also called volcanic rocks. Extrusive igneous rocks cool much more rapidly than intrusive rocks. There is little time for crystals to form, so extrusive igneous rocks have tiny crystals.Cooling rate and gas content create a variety of rock textures. Lavas that cool extremely rapidly may have a glassy texture. Those with many holes from gas bubbles have a vesicular texture.
Human Use of Igneous Rock
Igneous rocks have a wide variety of uses. One important use is as stone for buildings and statues. Granite is used for both of these purposes and is popular for kitchen countertops. Pumice is commonly used as an abrasive. Pumice is used to smooth skin or scrape up grime around the house. When pumice is placed into giant washing machines with newly manufactured jeans and tumbled, the result is “stone-washed” jeans. Ground up pumice stone is sometimes added to toothpaste to act as an abrasive material to scrub teeth. Peridotite is sometimes mined for peridot, a type of olivine that is used in jewelry. Diorite was used extensively by ancient civilizations for vases and other decorative artwork and is still used for art today.
- Dynamic Earth: Introduction to Physical Geography. Authored by: R. Adam Dastrup. Located at: http://www.opengeography.org/physical-geography.html. Project: Open Geography Education. License: CC BY-SA: Attribution-ShareAlike
- Tunnel View, Yosemite Valley. Authored by: David Iliff. Located at: https://commons.wikimedia.org/wiki/File:Tunnel_View,_Yosemite_Valley,_Yosemite_NP_-_Diliff.jpg. License: CC BY-SA: Attribution-ShareAlike
- Lava entering sea - Hawaii. Authored by: Jennifer Williams. Located at: https://commons.wikimedia.org/wiki/File:Lava_entering_sea_-_Hawaii.png. License: CC BY-SA: Attribution-ShareAlike
- Pahoehoe toe. Authored by: Hawaii Volcano Observatory. Provided by: USGS. Located at: http://hvo.wr.usgs.gov/multimedia/archive/2003/May/main.html. License: Public Domain: No Known Copyright
- Volcanos ed. Provided by: Hardwigg and USGS. Located at: https://commons.wikimedia.org/wiki/File:Volcanosed.svg. License: Public Domain: No Known Copyright

