6.10: Weathering
- Page ID
- 9987
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Weathering
Weathering is the gradual destruction of rock under surface conditions. Weathering may involve physical processes (called mechanical weathering) or chemical activity (called chemical weathering). Biological activity can also result in weathering that can be construed as mechanical, chemical, or both.
Weathering processes can begin long before rocks are exposed at the surface. This is true in most places on the earth surface where rocky outcrops (bedrock) is not exposed. In addition, weathering and erosion can take place simultaneously, perhaps most obviously in settings like rivers in flood, or waves crashing on a beach.
Mechanical Weathering
Mechanical weathering involves all processes that collectively break rocks into smaller pieces. Mechanical weathering includes all forms of mass wasting—a general name for processes by which soil and rock move downslope under the force of gravity. Mass wasting, a form of mechanical weathering, includes sudden events such as rock falls, landslides, slumps, and avalanches. These processes break "big pieces of rocks into smaller pieces."
Mechanical weathering can involve erosional grinding as fast-moving flood waters moves boulders and sediments down stream valleys and where wave action batters rocks into sand along a shoreline. Rocks are shattered by earthquakes and volcanic explosions, the expand and split when erosion unloads overburden on compressed rocks that were previously deeply buried. Rocks will split when water freezes and expands in cracks. Rocks exposed on the surface are subject to expansion and contraction caused by daily heating and cooling (particularly effective in arid environments). Mechanical weathering is also caused by organic activity—the breakdown and movement of rock and soil caused by expanding tree roots, burrowing, feeding activity, etc.
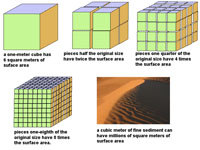
The mechanical breakdown of rocks increases the surface area (per unit area) increasing the available surface area where chemical weathering can take place (Figure 6.21).
Chemical Weathering
Chemical weathering involves the breakdown (decomposition, decay, and dissolution) of rock by chemical means. Dissolution is the action or process of dissolving or being dissolved, moving soluble components of materials into solution. Leaching is the process of dissolving and removing the soluble constituents of soil or rock near the land's surface. Water flowing under the influence of gravity carries dissolved materials away, ultimately adding to the saltiness of the oceans or they are deposited as salts, such a such as in an inland desert dry lake basin.
In most surface and near surface settings, mechanical and chemical weathering are taking place simultaneously.
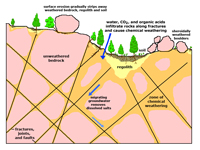 Figure 6.32. Weathering involves many processes occurring at or near the surface environment. Fractures allow water and air to penetrate into the bedrock allowing chemical weathering processes to take place.
Figure 6.32. Weathering involves many processes occurring at or near the surface environment. Fractures allow water and air to penetrate into the bedrock allowing chemical weathering processes to take place.
Weathering and erosion are continuous processes in the surface environment, enhanced by the presence of water. Water is commonly called the universal solvent because so many compounds can be dissolved in it.
The journey of sediments can take a long time! The migration of sediments from upland regions to the ocean basins can take a very long time. Sediments can eroded and re-deposited many times along the journey.
Fate of soluble components of rocks: formation of seawater
As rocks weather and erode, they loose their soluble elemental components, they dissolve in groundwater and surface runoff and are carried away, eventually reaching the ocean. The high level of salt in seawater comes from\ the weathering and erosion of rocks on the surface or the seafloor. Salts dissolved in water flowing off of the continents or water flowing through sediments or rocks underground. Evaporation concentrates salt in seawater. Salt concentrations higher than seawater occur isolated lake basins in arid regions, such as in North America's Great Basin region. Salts end up being concentrated as salts on dry lake beds—or brine in basins such as Mono Lake (CA) or the Great Salt Lake (Utah). Over billions of years, rivers and streams, and groundwater flowing into the oceans have contributed to the saltiness of seawater. Salt in seawater is concentrated by the evaporation of water back into the atmosphere. (See Why is the ocean salty?)
 |
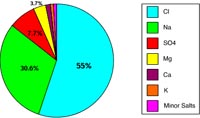 |
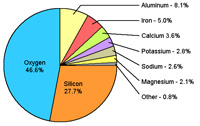
| Figure 6.37. Composition of crustal rocks, some elements are more soluble and others. | Figure 6.38. Elemental components of salts dissolved in seawater. |