12.4: The Carbon Cycle
- Page ID
- 13520
Introduction
The element carbon holds a distinctive place among the chemical elements: it can form covalent bonds with itself (these are called carbon–carbon double bonds and carbon–carbon triple bonds, depending upon how many of the bonding positions are involved) as well as with other elements, especially hydrogen, to form an enormous number of chemical compounds, called organic compounds.
The term “organic” here is a bit misleading: great numbers of such compounds are synthesized by organisms, but even greater numbers can be synthesized in the laboratory, from inorganic raw materials, without the involvement of organisms, living or dead. The field of chemistry devoted to the study of organic compounds is called organic chemistry.
Carbon also forms various inorganic compounds. In such compounds, carbon bonds covalently with oxygen. The two most important kinds of such carbon-containing inorganic compounds are carbon dioxide (CO2), invariably a gas under Earth-surface conditions, and carbonate minerals, mainly calcite, CaCO3, and dolomite, CaMg(CO3)2.
Carbon, as both organic compounds and inorganic compounds, is present at and near the Earth’s surface in a great variety of environments. The study of where carbon resides in and on the Earth, together with the modes and rates at which carbon moves from one to another of such environments, is known as the carbon cycle.
The concept of a “cycle” here carries the implication that there is an unceasing movement of carbon, in all of its various forms, in and on the Earth. In this respect the Earth can be viewed as a closed system with respect to carbon, in the sense that although the carbon undergoes a great variety of transformations the total amount does not change with time.
A full consideration of the Earth’s carbon cycle would necessitate casting a wide net: we would need to consider not only the Earth’s surface environment, including the atmosphere, the land surface, and the oceans, but also the Earth’s deep interior—because, by various processes, carbon becomes buried within the Earth, and, by various other processes, it is released from deep storage back into the surface environment. The carbon cycle is truly integrative, in the sense that its study draws upon a wide range of scientific disciplines: geology, biology, and climatology. Here we’ll concentrate on the Earth-surface part of the carbon cycle.
Elements of the Carbon Cycle
There are many reservoirs of carbon at and near the Earth’s surface. Table 12-2 shows the important carbon reservoirs, along with rough estimates of the mass of carbon in those reservoirs. (In ecology, the term biomass is used for the total mass of living material, in plants and animals. It can be expressed as mass per unit area in a particular region of the Earth’s surface, or as the total over the entire Earth.) You can see that the mass of carbon in the atmosphere, the oceans, and biomass is tiny in comparison to the carbon that resides in sedimentary rocks in the earth’s crust. Note also from Table 12-2 that the mass of carbon in the atmosphere is approximately equal to total biomass carbon.
In Figure 12-2 you can see the distinction between organic carbon and inorganic carbon. It’s useful to consider these two forms of carbon separately. It’s easier to deal with the latter than with the former.
As noted earlier, the two important forms of inorganic carbon are carbon dioxide and carbonate minerals and rocks. Carbon dioxide is present in the Earth’s atmosphere in an average concentration of close to 0.4 percent by volume.
Carbon is put into the atmosphere, in the form of carbon dioxide, mainly from respiration by organisms and by decomposition of organic matter, and also by burning of plant matter. Before the discovery of fire by human beings, burning was ignited by lightning strikes. Nowadays, of course, enormous quantities of carbon are put into the atmosphere by burning of fossil fuels. Carbon is also put into the atmosphere in the form of carbon dioxide released during volcanic eruptions.
Carbon is removed from the atmosphere mainly by photosynthesis by plants and by becoming dissolved in the oceans. Some of the added carbon dioxide from burning of fossil fuels results in increase in the concentration of carbon dioxide in the atmosphere, but that increase is in part offset by dissolution into the oceans and in part by increase in total plant biomass.
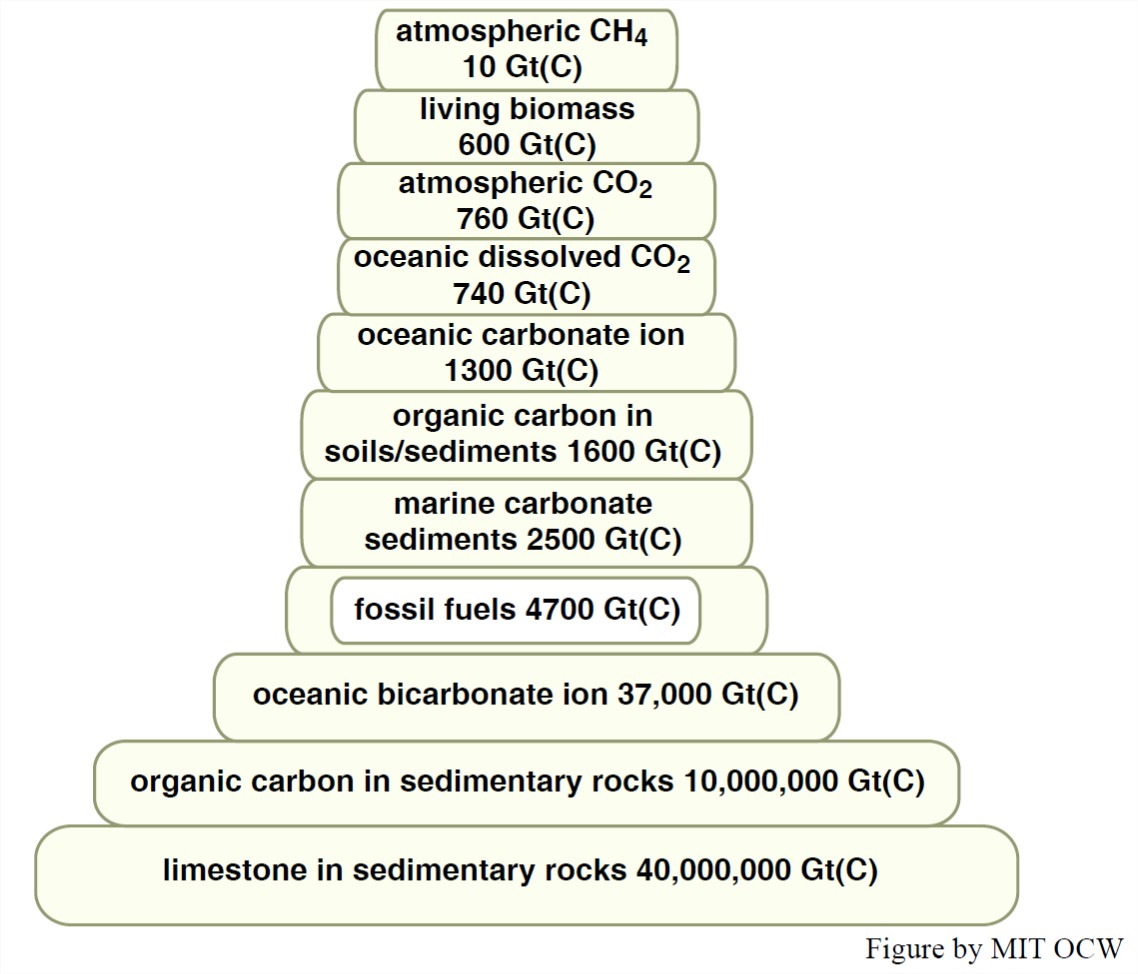
Incidentally, not all of the carbon in the atmosphere is in the form of carbon dioxide. Methane, CH4, is present in the atmosphere in concentrations of about 1.7 x 10-4 percent by volume. Methane is added to the atmosphere in two ways, mainly: upward seepage from shallow and deep subsurface, where methane is generated both from by the activity of microorganisms at shallow depths, and by complex reactions involving deeply buried organic matter at greater depths. Methane is unstable chemically in the atmosphere: it is converted to carbon dioxide and water vapor.
Inorganic carbon in the Earth’ crust is largely in the form of carbonate minerals calcite, aragonite, and dolomite. Enormous volumes of carbonate rocks, mainly limestone and dolostone, reside in the crust. As you learned in an earlier chapter, carbon in the form of carbon dioxide is released as carbonate rocks are weathered at the Earth’s surface. At the same time, carbonate minerals are precipitated, mainly in the shallow ocean, in places where the ocean water is oversaturated with respect to calcium carbonate. Most of that precipitation is in the tissues of a great variety of carbonate-secreting organisms, although some is precipitated inorganically.
The residence time of carbon in the atmosphere is about thirteen years. To figure that out, you need to know, in addition to the 760 Gton of carbon that’s present in the atmosphere, that the inflow of carbon into the atmosphere, and the outflow of carbon from the atmosphere, which from year to year are not far from being the same, is about 60 Gton carbon.


