10.9: Activity 2 - Carbon Dioxide
- Page ID
- 14701
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \) \( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)\(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\) \(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\)\(\newcommand{\AA}{\unicode[.8,0]{x212B}}\)
Name: ______________________________
Section: _____________________________
Student ID#:__________________________
- Draw a simple version of the carbon cycle. (Refer to your textbook for help.) Include the atmosphere and plants.
- What process of the carbon cycle is responsible for releasing CO2 into the soil atmosphere?
- How might this release of CO2 into the soil atmosphere affect the soil pH? Write a chemical reaction for this process.
- Rank the soils in order of probable amount of CO2 released, from the greatest amount released to the least amount released (begin with the soil with largest quantity of CO2 released. Briefly explain your answer.
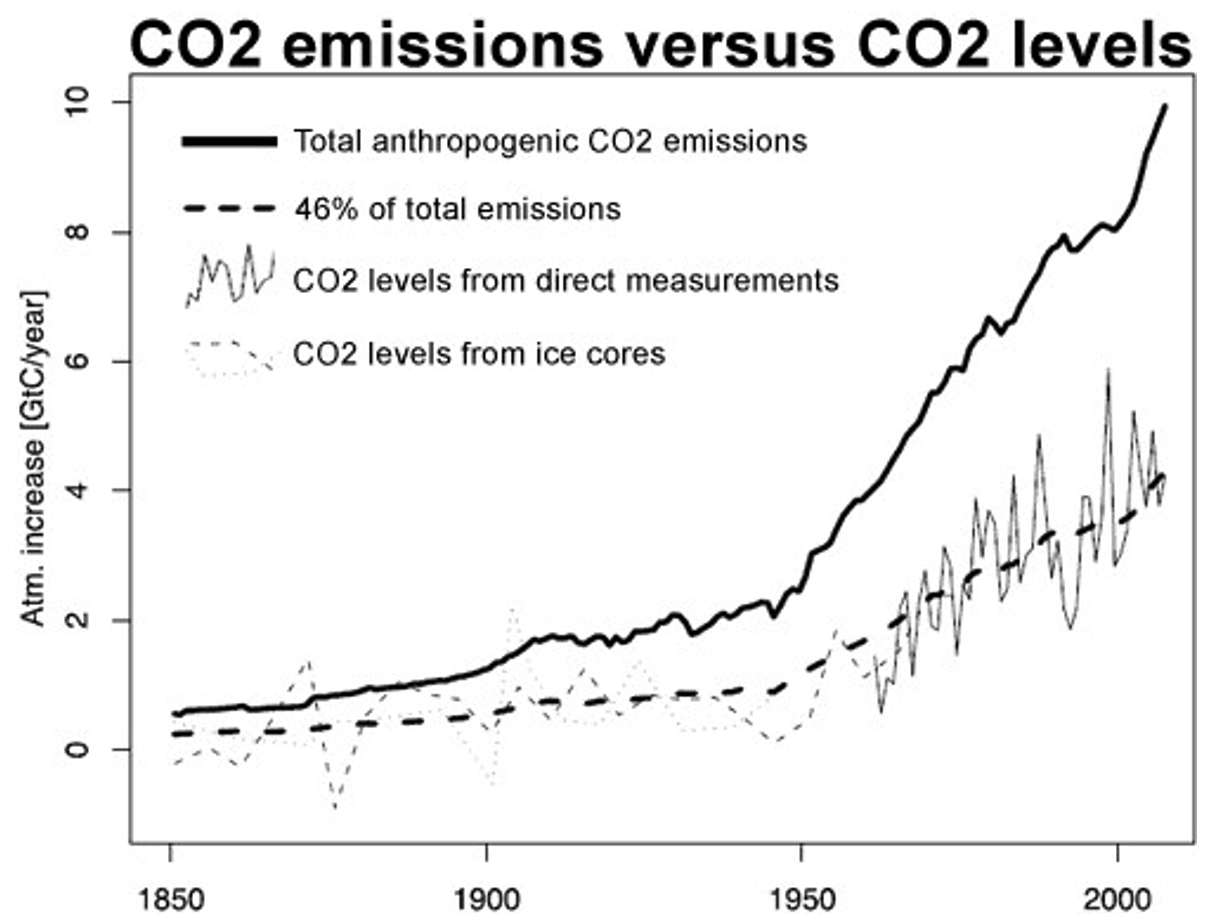
5. Increasing temperatures on earth is attributed to an increase in carbon dioxide, which traps heat via the greenhouse effect. The increased atmospheric carbon dioxide is due to the burning of fossil fuels. Explain why carbon dioxide levels in the world’s atmosphere has lagged behind emissions. Consider possible carbon sinks that may be keeping carbon in terrestrial and marine environments.


