3.4: Latent Heat and Heat Capacity
- Page ID
- 14931
These are the relevant physical properties of water and their significance--all of which are shaped by the hydrogen bonding between polar water molecules:
- Heat capacity (highest of all solids and liquids except NH3)
- Latent heat of fusion (highest except NH3)
- Latent heat of evaporation (highest of all substances)
- Thermal expansion (in the first section we showed that the temperature of maximum density decreases with increasing salinity)
- Conduction of heat (highest of all liquids)
- Surface tension (highest of all liquids)
- Dissolving power (the "universal solvent" dissolves more substances in greater quantities than any other liquid)
- Transparency (large absorption of radiant energy)
Heat capacity and latent heat are key properties that allow water (the oceans in particular) to play a major role in "regulating" Earth's climate. Water absorbs solar energy and releases it slowly; thus, larger bodies of water do not change temperature rapidly. Likewise, the high latent heat of vaporization (see below), indicates that when water vapor (derived from evaporation of water at the ocean's surface driven by solar energy receipt at low latitudes) condenses into liquid droplets at high elevations or high latitude, the latent heat is released into the environment. In Lesson 4, we will examine this role in more detail, and we have already alluded to the fact that large lakes can help buffer temperature changes.
Heat Capacity
A direct result of the hydrogen bond in water is the high heat capacity of water. As noted, a calorie is the amount of heat required to raise the temperature of 1 g of water 1 °C. The heat capacity of water compared to that of most other substances is great.
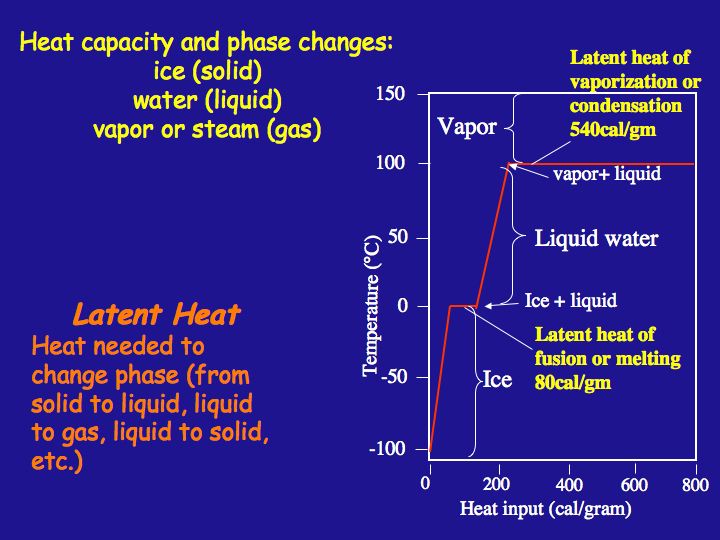
Latent Heats of Melting and Vaporization(refer to figure below)
Closely related to water's unusually high heat capacity are its high latent heat of melting and latent heat of vaporization. A solid converts to a liquid at a temperature called its freezing point and a liquid is changed to a gas at a temperature defined as its boiling point.
When changing the state of any substance, there may be no increase of temperature at that point where a change of state occurs even though heat is continuously being added. All the heat energy is being used to break all of the bonds (e.g. between polar water molecules) required to complete the change of state. The heat that is added to 1 g of a substance at the melting point to break the required bonds to complete the change of state from solid to liquid is the latent heat of melting. The heat applied to effect a change of state at the boiling point is the latent heat of vaporization. The amount of heat required to convert 1 g of ice to 1 g of water, 80 Cal, is termed the latent heat of melting, and it is higher for water than for any other commonly occurring substance. The amount of heat required to convert water to vapor, 540 Cal, is termed the latent heat of vaporization. The figure illustrates energy input to a given mass of water that begins as very cold ice and the temperature path that that mass of water takes with continued heat input. The path from condensation to cooling to ice formation returns energy to the environment.
Surface Tension
Next to mercury, water has the highest surface tension of all commonly occurring liquids. Surface tension is a manifestation of the presence of the hydrogen bond. Those molecules of water that are at the surface are strongly attracted to the molecules of water below them by their hydrogen bonds. If the diameter of the container is decreased, the combination of cohesion, which holds the water molecules together, and the adhesive attraction between the water molecules and the glass container will pull the column of water to great heights. This phenomenon is known as capillarity. This is, in part, what allows plants to stand up--when too much water is lost by evapo-transpiration, they wilt.
Credit:wt.kimiq.com(link is external)
Heat capacity and phase changes and latent heat.


