6.3.1: Equilibrium Between Crystals and Melt
- Page ID
- 18727
Every mineral has a characteristic melting temperature. This can be expected to lead to an orderly and predictable sequence of minerals crystallizing as magma cools and solidifies. It does, sort of. Complications arise because some minerals do not crystallize at a single temperature, but instead form from other minerals while reacting with magma. This is called incongruent melting. Further complications arise because minerals together may melt (and crystallize) at lower temperatures than if they if they were alone. This is called eutectic melting. For example, at 1 atm pressure, diopside melts at about 1391 ̊C and anorthite melts at 1553 ̊C. A rock composed of diopside and anorthite, however, will melt at 1278 ̊C.
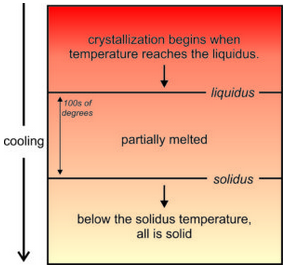
Crystallization of a cooling magma is a step-wise process with some minerals forming before others. So, crystallization occurs over a range of temperature. As seen in Figure 6.16, the first mineral crystals form at a temperature called the liquidus. Crystallization continues until the magma reaches the solidus temperature. Above the liquidus, all is melted. Below the solidus, all is solid. Between the two temperatures a rock is partially melted. The differences between liquidus and solidus temperatures can be 100s of degrees and is different for different composition magmas.
Different magmas produce different minerals, and different minerals crystallize at different temperatures. Consequently, composition is the key factor that determines the temperature at which crystallization begins. Temperatures measured in flowing lavas generally range from 900 to 1,100 °C, with higher temperatures corresponding to basaltic (mafic) lavas and lower temperatures to andesitic or rhyolitic (intermediate to silicic) lavas. For mafic rocks, the first minerals to crystallize are typically olivine, pyroxene, or plagioclase. For silicic rocks, the first crystals may be alkali feldspars or micas. Eventually, a magma completely solidifies, and the last drop of melt crystallizes at the solidus temperature. For some magmas, the solidus is well above 1,000 °C, but for granites and other silicic magmas it may be as low as 700 °C.


