2.4.1: Ionic Bonds
- Page ID
- 18292
Negatively and positively charged particles attract each other. Protons (positively charged) attract electrons (negatively charged) in atoms. Similarly, positively charged cations attract negatively charged anions, producing ionic bonds in minerals.
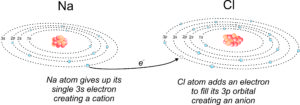
2.13 Ionic bonding to form halite (NaCl)
We call the process of losing electrons to become a cation oxidation. Gaining electrons, and becoming an anion, is reduction. The formation of cations and anions go hand in hand. Metals becoming cations donate electrons to non-metals becoming anions. The drawing shown in Figure 2.13 is a schematic showing how sodium and chlorine can combine to make the mineral halite (NaCl). First sodium (Na) and chlorine (Cl) react to produce the ions Na+ and Cl– (when a 3s electron from sodium is added to the 3p orbital in chlorine). Na is oxidized as Cl is reduced. These two ions then bond, producing NaCl.
Oxidation and reduction also occur when iron and oxygen combine to form hematite (Fe2O3). Two Fe0 atoms each donate 3 electrons that are picked up by three O0 atoms. So, the iron atoms become Fe3+ while the oxygen atoms become O2-. The ions may then bond ionically to produce (Fe2O3). Alternatively, iron sometimes oxidizes to Fe2+ and combines with oxygen to form the mineral wüstite (FeO). A third iron oxide mineral, magnetite (Fe3O4), contains both Fe2+ and Fe3+. Wüstite, hematite and magnetite are all minerals, but wüstite is not a common mineral on Earth (although it is found in meteorites) because under normal Earth surface conditions, iron easily oxidizes to become trivalent. The bonds in all three iron oxides, however, are not entirely ionic; they are partially covalent (discussed later in this chapter).


