2.2.5: Periods
- Page ID
- 18290
In atoms, electrons are in orbitals of different energies around atomic nuclei. Electrons are always moving, and orbitals are regions around a nucleus where electrons are likely to be found. We designate an orbital using a number and a letter, for example 1s or 1p – the number refers to a specific electron shell and the letter to a subshell. S subshells can hold up to 2 electrons, p subshells up to 6 electrons, d subshells up to 10 electrons, and f subshells up to 14 electrons. The table below lists, from lowest to highest energy, the orbitals and the maximum number of electrons they can hold. s orbitals are spherical, p orbitals are dumbbell shaped, d and f orbitals have more complicated shapes. The shape of an atom’s outermost occupied orbital is often important because it can affect mineral properties such as color. But, for simplicity, in the drawings below we show all orbitals as circular.
| Electron Orbitals | |||||||||||||||||||
| orbital | 1s | 2s | 2p | 3s | 3p | 4s | 3d | 4p | 5s | 4d | 5p | 6s | 4f | 5d | 6p | 7s | 5f | 6d | 7p |
| max # electrons | 2 | 2 | 6 | 2 | 6 | 2 | 10 | 6 | 2 | 10 | 6 | 2 | 14 | 10 | 6 | 2 | 14 | 10 | 6 |
| increasing energy ➔ | |||||||||||||||||||
In a neutral (non-ionized) atom, the number of electrons is equal to the atom’s atomic number. So, a hydrogen atom (atomic number 1) contains one electron, a helium atom (atomic number 2) contains two electrons, a lithium atom (atomic number 3) contains three electrons, and so forth to high atomic number. Electrons populate orbitals sequentially, filling the lowest energy orbitals first and proceeding to higher energy orbitals. Thus, hydrogen has one electron in a 1s orbital. Helium has two electrons in a 1s orbital, lithium has two electrons in a 1s orbital and one electron in a 2s orbital, etc.
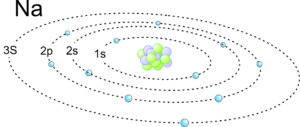
2.9 Electron orbits in a sodium atom
For an example of electron configuration in a heavier element, consider sodium. This schematic drawing in Figure 2.9 shows orbitals and electrons in a sodium atom. The electrons are orbiting a nucleus that contains protons and neutrons (but they are not all visible). Sodium has atomic number 11, so a neutral atom has 11 protons and 11 electrons. Natural sodium also has 12 neutrons. The electrons fill the 1s, 2s, and 2p orbitals. One additional electron is in the 3s orbital.
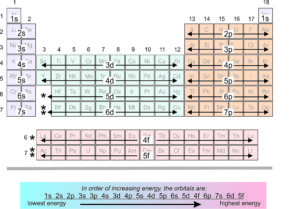
2.10 Electron orbitals of valence electrons
We call electrons that contribute to chemical bonding valence electrons. These electrons are generally in the outermost electron shells (but may be in an inner shell for transition metals). This chart (Figure 2.10) shows the orbitals of valence electrons for different elements. Elements in the same period (row) of the periodic table have their valence electrons in the same s or p shell, or (for transition elements) in a d orbital of the next lower shell.
The first period contains only two elements (H and He), while the second and third have eight each. Electrons for second and third period elements are in 2s, 2p, 3s, or 3p orbitals. The fourth through seventh periods contain some elements with valence electrons in s or p orbitals, but also contain 20 elements having valence electrons in d-orbitals. The sixth and seventh periods contain elements with valence electrons in s, p, d, or f orbitals. The lanthanides and actinides have valence electrons in f orbitals. Thus, as seen in the chart above, elements with high atomic number (compared with elements of low atomic number) have valence electrons in higher-energy orbitals.


