3.6: Getting Ready for Chapter 3
- Page ID
- 15855
Chapter 2 set the stage for a comprehensive survey of the earth system. We'll begin with the atmosphere, the subject of chapter 3. The structure and composition of our present day atmosphere has been relatively stable for millions of years. During the depths of the ice ages, the carbon dioxide content of the atmosphere especially decreased which helped keep air temperatures cool. But levels of oxygen have remained fairly constant, even through the ice ages for the last 50 million years or so. But, the composition of the atmosphere especially greenhouse gases like carbon dioxide and methane are changing at rates much greater than they did in the past. These changes are in part due to natural forces, but increasingly are due to the activities of humans.
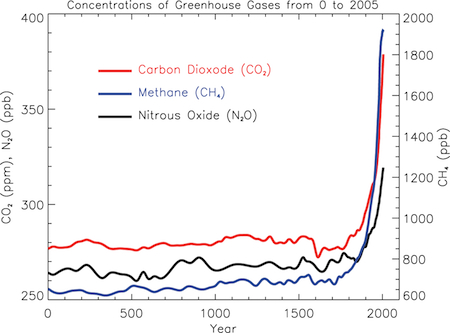
Concentrations of Greenhouse Gases from 0 to 2005
(Courtesy IPCC)
In Chapter 3 we'll examine the composition of the atmosphere and the importance of particular gases to the functioning of the earth system. We'll look out the structure of the atmosphere and how scientists have subdivided it based on vertical temperature patterns and the function of the different atmospheric layers. We'll also peek into the future to see what changes we can expect in our atmosphere as it changes in response to the activities of an ever-increasing population and an evolving world economy.
What you should already know ...
Chapter 3 explores the the composition and structure of the atmosphere. The atmosphere interacts with the other subsystems of the earth system. Have a good understanding of the atmosphere as a system. Biogeochemical cycles are the means whereby substances are transformed and transported through the earth system. The composition of the atmosphere is directly influenced by these cycles.
- The atmosphere is considered
- an open system
- a closed system
- an isolated system
- None of the above
- The early atmosphere was a product of _____.
- accretion
- tectonic plate movement
- outgassing
- none of the above
- A change in a system property that encourages further system change is called
- a positive feedback
- a negative feedback
- a threshold
- an equilibrium trigger
- Evaporation transfers _____ from the hydrosphere to the atmosphere.
- heat
- mass
- heat and mass
- none of the above
- ______ is a feature of systems where no additional forcing is required for a large climate change and impact to occur.
- A positive feedback
- A tipping point
- A negative feedback
- None of the above
- Which of the following would act to keep a system in a state of equilibrium?
- a positive feedback
- a negative feedback
- a threshold
- an equilibrium trigger
- _______ is responsible for most of the oxygen found in the atmosphere.
- Transpiration
- Photosynthesizing vegetation
- Rock weathering
- Animal respiration
- ______ is responsible for most of the water vapor entering the atmosphere.
- Plant transpiration
- Precipitation
- Evaporation of water from land
- Evaporation of water from the oceans
- Though the atmosphere's temperature has varied throughout geologic history, much of the time it has been considered to be
- in a state of dynamic equilibrium
- in steady-state equilibrium
- in a non-equilibrium state
- in a geo-equilibrium state
- The heat to raise the temperature of the air ultimately comes from ______ sources of energy.
- endogenic
- exogenic
- both endogenic and exogenic
- none of the above
Answer
-
- A
- C
- A
- C
- B
- B
- B
- D
- A
- B
About your score ....
If you scored 80% or above, Great! ... start reading the chapter.
If you scored 70% to 80% you should consider reviewing the previous chapter.
If you scored less than 70% you should consider reviewing the previous chapter and seeking help from your instructor.


